Metabolomic Analysis of Actinic Keratosis and SCC Suggests a Grade-Independent Model of Squamous Cancerization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Metabolomic Results
2.1.1. PCA Analysis
2.1.2. Quantitative Analysis
2.1.3. Cluster Heat Map
2.1.4. ANOVA Analysis
2.2. Histopathological Results
3. Discussion
3.1. Increased Metabolism in AK and SCC as Compared with Healthy Skin and Different Metabolic Profile among AKI, AKII, AKIII and SCC
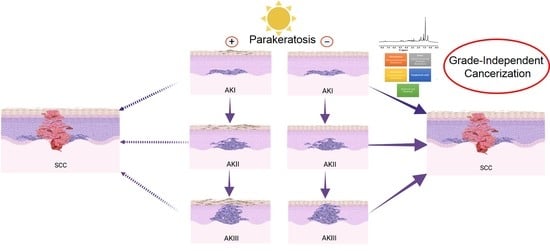
3.2. Histological Association between AK I and SCC
4. Material and Methods
4.1. Study Setting and Design
4.2. Sample Storage and Preparation
4.3. Metabolomic Study
4.3.1. Patients
4.3.2. HR-MAS NMR Measurements
4.3.3. Data Processing
4.3.4. Statistical Analysis
4.4. Histopathology Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Rubió-Casadevall, J.; Hernandez-Pujol, A.; Ferreira-Santos, M.; Morey-Esteve, G.; Vilardell, L.; Osca-Gelis, G.; Vilar-Coromina, N.; Marcos-Gragera, R. Trends in incidence and survival analysis in non-melanoma skin cancer from 1994 to 2012 in Girona, Spain: A population-based study. Cancer Epidemiol. 2016, 45, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Schnoor, M.; Eisemann, N.; Katalinic, A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J. Dtsch. Dermatol. Ges. 2015, 13, 788–797. [Google Scholar] [CrossRef]
- Perera, E.; Gnaneswaran, N.; Staines, C.; Win, A.K.; Sinclair, R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas. J. Dermatol. 2015, 56, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelbronn, M.A.; Mullins, D.L.; Ramos-Caro, F.A.; Flowers, F.P. Frequency of pre-existing actinic keratosis in cutaneous squamous cell carcinoma. Int. J. Dermatol. 1998, 37, 677–681. [Google Scholar] [CrossRef]
- Dika, E.; Fanti, P.A.; Misciali, C.; Vaccari, S.; Crisman, G.; Barisani, A.; Baraldi, C.; Ribero, S.; Patrizi, A. Risk of skin cancer development in 672 patients affected by actinic keratosis. G. Ital. Dermatol. Venereol. 2016, 151, 628–633. [Google Scholar] [PubMed]
- Quaedvlieg, P.J.; Tirsi, E.; Thissen, M.R.; Krekels, G.A. Actinic keratosis: How to differentiate the good from the bad ones? Eur. J. Dermatol. 2006, 16, 335–339. [Google Scholar] [PubMed]
- Cockerell, C.J. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J. Am. Acad. Dermatol. 2000, 42, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, C.J.; Wharton, J.R. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J. Drugs Dermatol. 2005, 4, 462–467. [Google Scholar]
- Fernández-Figueras, M.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Korgavkar, K.; Weinstock, M.A. Current perspective on actinic keratosis: A review. Br. J. Dermatol. 2017, 177, 350–358. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2021, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Peng, Y.; Nie, Y.; Yu, J.; Wong, C.C. Microbial Metabolites in Colorectal Cancer: Basic and Clinical Implications. Metabolites 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Rajani, C.; Jia, W. The Distinctive Serum Metabolomes of Gastric, Esophageal and Colorectal Cancers. Cancers 2021, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- Saorin, A.; Di Gregorio, E.; Miolo, G.; Steffan, A.; Corona, G. Emerging Role of Metabolomics in Ovarian Cancer Diagnosis. Metabolites 2020, 10, 419. [Google Scholar] [CrossRef]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metabolomics 2019, 15, 22. [Google Scholar] [CrossRef]
- Righi, V.; Tarentini, E.; Mucci, A.; Reggiani, C.; Rossi, M.C.; Ferrari, F.; Casari, A.; Magnoni, C. Field cancerization therapy with ingenol mebutate contributes to restoring skin-metabolism to normal-state in patients with actinic keratosis: A metabolomic analysis. Sci. Rep. 2019, 9, 11515. [Google Scholar]
- Ackerman, A.B.; Mones, J.M. Solar (actinic) keratosis is squamous cell carcinoma. Br. J. Dermatol. 2006, 155, 9–22, Erratum in: Br. J. Dermatol. 2006, 155, 500. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Poulsen, T.; Wulf, H.C. Actinic keratoses contiguous with squamous cell carcinomas are mostly non-hyperkeratotic and with severe dysplasia. J. Clin. Pathol. 2021, 207497. [Google Scholar] [CrossRef]
- Arenberger, P.; Arenbergerova, M. New and current preventive treatment options in actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 5), 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadavand, M.A.; Mayer, D.; Chen, W.; Wnorowski, A.; Siddiqui, M.M. Role of metabolic imaging in diagnosis of primary, metastatic, and recurrent prostate cancer. Curr. Opin. Oncol. 2020, 32, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Oshima, N.; Krishna, M.C.; Gillies, R.J. Direct and indirect assessment of cancer metabolism explored by MRI. NMR Biomed. 2019, 32, e3966. [Google Scholar] [CrossRef]
- Serkova, N.J.; Eckhardt, S.G. Metabolic Imaging to Assess Treatment Response to Cytotoxic and Cytostatic Agents. Front. Oncol. 2016, 6, 152. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phospahtidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef]
- Randhawa, M.; Sangar, V.; Tucker-Samaras, S.; Southall, M. Metabolic signature of sun exposed skin suggests catabolic pathway overweighs anabolic pathway. PLoS ONE 2014, 9, e90367. [Google Scholar] [CrossRef]
- Kurmi, K.; Haigis, M.C. Nitrogen Metabolism in Cancer and Immunity. Trends Cell Biol. 2020, 30, 408–424. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef] [Green Version]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef] [Green Version]
- Labuschagne, C.F.; van den Broek, N.J.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bachhawat, A.K. Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role inangiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef]
- Stockfleth, E.; Ferrandiz, C.; Grob, J.J.; Leigh, I.; Pehamberger, H.; Kerl, H. Development of a treatment algo- rithm for actinic keratosis: A European Consensus. Eur. J. Dermatol. 2008, 18, 651–659. [Google Scholar] [CrossRef]
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Stockfleth, E.; Szeimies, R.M.; Dirschka, T. Actinic keratoses show variable histological basal growth—A proposed classification adjustment. J Eur Acad Dermatol Venereol. 2018, 32, 745–751. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell 1980, 22, 629–632. [Google Scholar] [CrossRef]
- Alonso-Lecue, P.; De Pedro, I.; Coulon, V.; Molinuevo, R.; Lorz, C.; Segrelles, C.; Ceballos, L.; López-Aventín, D.; García-Valtuille, A.; Bernal, J.M.; et al. Inefficient differentiation response to cell cycle stress leads to genomic instability and malignant progression of squamous carcinoma cell. Cell Death Dis. 2017, 8, e2901. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Jorizzo, J.J.; Schaffer, J.V.; Callen, J.P.; Cerroni, L.; Heymann, W.R.; Hruza, G.J.; Mancini, A.J.; McGrath, J.; Patterson, J.W.; et al. Dermatology, 3rd ed.; Elsevier: London, UK, 2012. [Google Scholar]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours. In WHO Classification of Tumours, 4th ed.; IARC Pubblications: Lyon, France, 2018; Volume 11. [Google Scholar]
- Schenetti, L.; Mucci, A.; Parenti, F.; Cagnoli, R.; Righi, V.; Tosi, M.R.; Tugnoli, V. HR-MAS NMR spectroscopy in the characterization of human tissues: Application to healthy gastric mucosa. Concepts Magn. Reson. Part A 2006, 28A, 430–443. [Google Scholar] [CrossRef]
- Righi, V.; Cavallini, N.; Valentini, A.; Pinna, G.; Pavesi, G.; Rossi, M.C.; Puzzolante, A.; Mucci, A.; Cocchi, M. A metabolomic data fusion approach to support gliomas grading. NMR Biomed. 2020, 33, e4234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, M.G.; Zektzer, A.S.; Tabatabai, Z.L.; Simko, J.; Jarso, S.; Keshari, K.R.; Schmitt, L.; Carroll, P.R.; Shinohara, K.; Vigneron, D.B.; et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn. Reson. Med. 2006, 55, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| f-Value | p-Value | log10 (p-Value) | FDR | Fisher’s LSD | |
|---|---|---|---|---|---|
| Glutamate | 5.6949 | 0.001004 | 2.9983 | 0.015728 | 1-0; 2-0; 3-0; 4-0; 4-1; 4-2 |
| Ethanolamine | 5.5884 | 0.001138 | 2.9438 | 0.015728 | 3-0; 4-0; 4-1; 3-2; 4-2 |
| Glutamine | 5.3167 | 0.001573 | 2.8033 | 0.015728 | 1-0; 2-0; 3-0; 4-0 |
| Glutathione | 4.9945 | 0.002321 | 2.6343 | 0.017409 | 1-0; 3-0; 4-0; 2-1; 4-2; 3-1; 3-4 |
| Methionine | 4.2611 | 0.005762 | 2.2395 | 0.034569 | 1-0; 2-0; 3-0; 4-0; 4-2 |
| Pyroglutamic acid | 4.0771 | 0.007273 | 2.1383 | 0.036367 | 3-0; 4-0; 3-1; 3-2; 3-4; 4-2 |
| Lactate | 3.7253 | 0.011419 | 1.9424 | 0.045287 | 3-0; 4-0 |
| Threonine | 3.6821 | 0.012076 | 1.9181 | 0.045287 | 3-0; 4-0;3-1; 3-2; 4-2; 4-3 |
| Myo-inositol | 3.3984 | 0.017471 | 1.7577 | 0.058237 | 4-0; 4-1; 4-2; 4-3 |
| Glycine | 3.1441 | 0.024413 | 1.6124 | 0.067903 | 3-0; 4-0; 3-2; 3-4 |
| Taurine | 2.97 | 0.030749 | 1.5122 | 0.076389 | 1-0; 2-0; 3-0; 4-0 |
| Choline | 2.7793 | 0.039645 | 1.4018 | 0.076389 | 4-0; 4-1; 4-2; 4-3 |
| Serine | 2.7589 | 0.040741 | 1.39 | 0.076389 | 3-0; 4-0; 3-1 3-2; 3-4; 4-2 |
| AK I (n = 63) | AK II (n = 77) | AK III (n = 30) | Total (n = 170) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Gender | |||||||||
| Female | 16 | 25.4 | 25 | 32.5 | 5 | 16.7 | 46 | 27.1 | 0.220 |
| Male | 47 | 74.6 | 51 | 66.2 | 25 | 83.3 | 123 | 72.4 | |
| Site | 0 | ||||||||
| Limbs | 13 | 20.6 | 11 | 14.3 | 2 | 6.7 | 26 | 15.3 | 0.076 |
| Head and neck | 49 | 77.8 | 66 | 85.7 | 26 | 86.7 | 141 | 82.9 | |
| Trunk | 1 | 1.6 | 0 | 0.0 | 2 | 6.7 | 3 | 1.8 | |
| Associated SCC | 20 | 31.7 | 18 | 23.4 | 7 | 23.3 | 45 | 26.5 | <0.001 |
| SCC grade | |||||||||
| G1 | 3 | 15.0 | 4 | 22.2 | 3 | 42.9 | 10 | 22.2 | 0.064 |
| G2 | 15 | 75.0 | 6 | 33.3 | 3 | 42.9 | 24 | 53.3 | |
| G3 | 1 | 5.0 | 2 | 11.1 | 1 | 14.3 | 4 | 8.9 | |
| in situ | 1 | 5.0 | 6 | 33.3 | 0 | 0.0 | 7 | 15.6 | |
| SCC characteristics | |||||||||
| Breslow thickness, mm (median (IQR)) | 1.8 (0.3–5) | 0.5 (0.3–1.2) | 0.6 (0.4–0.8) | 0.9 (0.5–3.5) | 0.005 | ||||
| Clark level for SCC | |||||||||
| 2 | 4 | 21.1 | 9 | 75.0 | 5 | 71.4 | 18 | 47.4 | 0.062 |
| 3 | 5 | 26.3 | 1 | 8.3 | 1 | 14.3 | 7 | 18.4 | |
| 4 | 4 | 21.1 | 0 | 0.0 | 1 | 14.3 | 5 | 13.2 | |
| 5 | 6 | 31.6 | 2 | 16.7 | 0 | 0.0 | 8 | 21.1 | |
| Perineural invasion | 0 | 0.0 | 1 | 6.7 | 0 | 0.0 | 1 | 2.4 | 0.411 |
| Ulceration | 6 | 31.6 | 4 | 57.1 | 3 | 42.9 | 13 | 31.7 | 0.749 |
| Metastasis | 1 | 7.1 | 0 | 0.0 | 0 | 0.0 | 1 | 3.2 | 0.534 |
| Recurrence | 1 | 7.1 | 1 | 9.1 | 0 | 0.0 | 2 | 6.5 | 0.759 |
| AK characteristics | |||||||||
| Parakeratosis | 37 | 58.7 | 68 | 88.3 | 29 | 96.7 | 134 | 78.8 | <0.001 |
| Orthokeratosis | 16 | 25.4 | 34 | 44.2 | 12 | 40.0 | 62 | 36.5 | 0.065 |
| Acantholysis | 3 | 4.8 | 15 | 19.5 | 13 | 43.3 | 31 | 18.2 | <0.001 |
| Clear cells | 24 | 38.1 | 18 | 23.4 | 9 | 30.0 | 51 | 30.0 | 0.167 |
| Hyperplasia | 12 | 19.0 | 43 | 55.8 | 23 | 76.7 | 78 | 45.9 | <0.001 |
| Elastosis | 63 | 100 | 77 | 100 | 29 | 96.7 | 169 | 99.4 | 0.096 |
| Atrophy | 29 | 46.0 | 35 | 45.5 | 12 | 40.0 | 76 | 44.7 | 0.847 |
| Hypertrophy, grade | |||||||||
| 0 | 38 | 60.3 | 34 | 44.2 | 12 | 40.0 | 84 | 49.4 | 0.203 |
| 1 | 25 | 39.7 | 42 | 54.5 | 18 | 60.0 | 85 | 50.0 | |
| 2 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 1 | 0.6 | |
| Inflammation, grade | |||||||||
| 0 | 16 | 25.4 | 7 | 9.1 | 1 | 3.3 | 24 | 14.1 | 0.029 |
| 1 | 27 | 42.9 | 32 | 41.6 | 11 | 36.7 | 70 | 41.2 | |
| 2 | 14 | 22.2 | 26 | 33.8 | 12 | 40.0 | 52 | 30.6 | |
| 3 | 6 | 9.5 | 12 | 15.6 | 6 | 20.0 | 24 | 14.1 | |
| Pigmentation | 17 | 27.0 | 15 | 19.5 | 5 | 16.7 | 37 | 21.8 | 0.427 |
| Ulceration | 5 | 7.9 | 1 | 1.3 | 5 | 16.7 | 11 | 6.5 | 0.034 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Parakeratosis | 0.25 | (0.11–0.54) | 0.001 | 0.19 | (0.08–0.46) | <0.001 |
| Orthokeratosis | 0.74 | (0.35–1.49) | 0.385 | |||
| Acantholysis | 0.47 | (0.17–1.32) | 0.156 | |||
| Clear cells | 0.68 | (0.31–1.49) | 0.344 | |||
| Hyperplastic AK | 0.72 | (0.36–1.44) | 0.357 | |||
| Elastosis | 1 | empty | ||||
| Atrophy | 0.52 | (0.25–1.06) | 0.076 | |||
| Hypertrophy | ||||||
| Absent | ref. | 0.045 | ref. | |||
| Present | 2.01 | (1.01–3.97) | 2.75 | (1.28–5.91) | 0.009 | |
| Inflammation, grade | ||||||
| 0 | ref. | |||||
| 1 | 0.69 | (0.25–1.88) | 0.473 | |||
| 2 | 0.66 | (0.23–1.91) | 0.452 | |||
| 3 | 0.66 | (0.19–2.33) | 0.526 | |||
| Pigmentation | 0.86 | (0.37–2.01) | 0.738 | |||
| Ulceration | 0.24 | (0.02–1.93) | 0.181 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righi, V.; Reggiani, C.; Tarentini, E.; Mucci, A.; Paganelli, A.; Cesinaro, A.M.; Mataca, E.; Kaleci, S.; Ferrari, B.; Meleti, M.; et al. Metabolomic Analysis of Actinic Keratosis and SCC Suggests a Grade-Independent Model of Squamous Cancerization. Cancers 2021, 13, 5560. https://doi.org/10.3390/cancers13215560
Righi V, Reggiani C, Tarentini E, Mucci A, Paganelli A, Cesinaro AM, Mataca E, Kaleci S, Ferrari B, Meleti M, et al. Metabolomic Analysis of Actinic Keratosis and SCC Suggests a Grade-Independent Model of Squamous Cancerization. Cancers. 2021; 13(21):5560. https://doi.org/10.3390/cancers13215560
Chicago/Turabian StyleRighi, Valeria, Camilla Reggiani, Elisabetta Tarentini, Adele Mucci, Alessia Paganelli, Anna Maria Cesinaro, Ema Mataca, Shaniko Kaleci, Barbara Ferrari, Marco Meleti, and et al. 2021. "Metabolomic Analysis of Actinic Keratosis and SCC Suggests a Grade-Independent Model of Squamous Cancerization" Cancers 13, no. 21: 5560. https://doi.org/10.3390/cancers13215560
APA StyleRighi, V., Reggiani, C., Tarentini, E., Mucci, A., Paganelli, A., Cesinaro, A. M., Mataca, E., Kaleci, S., Ferrari, B., Meleti, M., & Magnoni, C. (2021). Metabolomic Analysis of Actinic Keratosis and SCC Suggests a Grade-Independent Model of Squamous Cancerization. Cancers, 13(21), 5560. https://doi.org/10.3390/cancers13215560