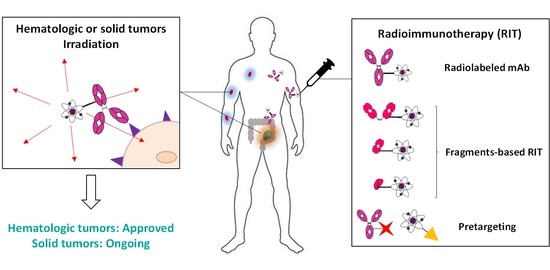
Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Last Ten Years Publications Involving RIT Protocols
2.1. Combination for Hematologic Malignancies: Modest Outcomes-Based RIT?
2.1.1. Therapeutic Strategies Studied
2.1.2. Low-Grade B-NHL
2.1.3. Aggressive B-NHL
2.1.4. Other Hematologic Diseases
2.1.5. Modest Efficacy?
2.2. RIT for Solid Tumors: A Viable Strategy?
2.2.1. Overview of the Last Ten Years of RIT Involving Full-Length Antibodies
2.2.2. Combination RIT
| Target/Vector | Isotope | Clinical Phase 1 | n 2 | Association 3 | Cold mAb (+/−) | Fractionation (+/−) | PFS (Months) or X-Year PFS (%) | OS (Months) or X-Year OS (%) | NCT | Year of Publication | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2-expressing breast cancer, peritoneal carcinomatosis or gastric cancer | |||||||||||
| HER2/Trastuzumab | Pb-212 | I | 16 | − | + | − | − | − | − | 2014 | [73] |
| Lu-177 | I | 10 | − | + | − | − | − | − | 2017 | [74] | |
| Medulloblastoma and neuroblastoma | |||||||||||
| B7H3/Omburtamab | I-131 | Retro | 94 | (R) | − | + | − | − | NCT00445965 NCT00089245 | 2015 | [71] |
| GD2 /3F8 | II | 43 | - | − | + | 11 | 24.9 | − | 2018 | [75] | |
| Metastatic colorectal cancer | |||||||||||
| A33/huA33 | I-131 | I | 19 | (C) | − | − | 5 | 28.7 | NCT00291486 | 2014 | [76] |
| CEA/cT84.66 | Y-90 | I/II | 16 | (C) | − | − | 9.6 | 41.2 | − | 2017 | [77] |
| CEA/Labetuzumab | I-131 | II | 63 | - | − | − | 16 | 55 | NCT27763687 | 2017 | [78] |
| Metastatic melanoma | |||||||||||
| MSCP/cDTPA-9.2.27 | Bi-213 | I | 38 | - | − | + | 20.4 | − | − | 2011 | [79] |
| Metastatic pancreatic cancer | |||||||||||
| hPAM4 (MUC-1)/Clivatuzumab tetraxetan | Y-90 | I | 21 | - | − | + | 1.3 | 4.3 | NCT00603863 | 2011 | [80] |
| I | 42 | (C) | − | + | − | 7.7 | NCT01956812 | 2012 | [81] | ||
| Ib | 58 | (C) | − | + | − | 7.9 (A) vs. 3.4 (B) | NCT01956812 | 2015 | [82] | ||
| Metastatic prostate cancer | |||||||||||
| PSMA/J591 | Lu-177 | II | 47 | - | + | − | − | 22.2 vs. 11.4 | NCT00195039 | 2013 | [83] |
| I/II | 49 | - | − | − | − | 42.3 | NCT00538668 | 2019 | [84] | ||
| I | 15 | (C) | − | − | − | − | NCT00916123 | 2020 | [85] | ||
| I/II | 6 | - | − | + | − | − | NCT00538668 | 2020 | [86] | ||
| Metastatic renal cell carcinomaz | |||||||||||
| CAIX/Girentuximab | Lu-177 | I | 23 | - | − | + | 11.1 | 25.3 | − | 2013 | [87] |
| II | 14 | (C + R) | − | + | − | − | NCT02002312 | 2016 | [88] | ||
| Non-small cell lung cancer | |||||||||||
| DNA/chTNT | I-131 | 96 | (C + R+Pmc) | − | − | − | 23–29.1 | − | 2016 | [89] | |
| Sarcoma | |||||||||||
| FZD10/OTSA-101 | Y-90 | I | 20 | - | − | + | PR in 3/8 | − | NCT01469975 | 2018 | [90] |
| B7H3/Omburtamab | I-131 | I/II | 52 | - | − | − | − | − | NCT01099644 | 2020 | [91] |
2.2.3. Choice of the Targets/mAbs/Radionuclides
3. Alternatives to Conventional RIT and Prospects
3.1. Fragments-Based RIT
3.1.1. Fab′
3.1.2. F(ab′)2
3.1.3. ScFv
3.1.4. Limitations and Prospects of Antibody Fragments
3.2. Pretargeted Radioimmunotherapy (PRIT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bosch, F.; Rosich, L. The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize. Pharmacology 2008, 82, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Teulon, I.; Lozza, C.; Pelegrin, A.; Vives, E.; Pouget, J.P. General overview of radioimmunotherapy of solid tumors. Immunotherapy 2013, 5, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Herrero Alvarez, N.; Bauer, D.; Hernandez-Gil, J.; Lewis, J.S. Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16, 2909–2941. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.P.; Navarro-Teulon, I.; Bardies, M.; Chouin, N.; Cartron, G.; Pelegrin, A.; Azria, D. Clinical radioimmunotherapy—The role of radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef]
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox Signal. 2018, 29, 1447–1487. [Google Scholar] [CrossRef] [PubMed]
- Kersten, M.J. Radioimmunotherapy in follicular lymphoma: Some like it hot. Transfus Apher Sci 2011, 44, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Derenzini, E.; Pellegrini, C.; Rigacci, L.; Fabbri, A.; Gandolfi, L.; Argnani, L.; Casadei, B.; Pulsoni, A.; Gobbi, M.; et al. Long-term efficacy and toxicity results of the FLUMIZ trial (fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in untreated follicular lymphoma). Ann. Oncol. 2012, 23, 805–807. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Tani, M.; Pulsoni, A.; De Renzo, A.; Stefoni, V.; Broccoli, A.; Montini, G.C.; Fina, M.; Pellegrini, C.; Gandolfi, L.; et al. A phase II trial of short course fludarabine, mitoxantrone, rituximab followed by (9)(0)Y-ibritumomab tiuxetan in untreated intermediate/high-risk follicular lymphoma. Ann. Oncol. 2012, 23, 415–420. [Google Scholar] [CrossRef]
- Scholz, C.W.; Pinto, A.; Linkesch, W.; Linden, O.; Viardot, A.; Keller, U.; Hess, G.; Lastoria, S.; Lerch, K.; Frigeri, F.; et al. (90)Yttrium-ibritumomab-tiuxetan as first-line treatment for follicular lymphoma: 30 months of follow-up data from an international multicenter phase II clinical trial. J. Clin. Oncol. 2013, 31, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.; Kassar, M.; Venugopal, P.; Shammo, J.M.; Fung, H.C.; Bayer, R.; O’Brien, T.; Gregory, S.A. Safety and efficacy of combination therapy with fludarabine, mitoxantrone, and rituximab followed by yttrium-90 ibritumomab tiuxetan and maintenance rituximab as front-line therapy for patients with follicular or marginal zone lymphoma. Clin. Lymphoma Myeloma Leuk. 2011, 11, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Khouri, I.F.; Saliba, R.M.; Erwin, W.D.; Samuels, B.I.; Korbling, M.; Medeiros, L.J.; Valverde, R.; Alousi, A.M.; Anderlini, P.; Bashir, Q.; et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood 2012, 119, 6373–6378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illidge, T.M.; Mayes, S.; Pettengell, R.; Bates, A.T.; Bayne, M.; Radford, J.A.; Ryder, W.D.; Le Gouill, S.; Jardin, F.; Tipping, J.; et al. Fractionated (9)(0)Y-ibritumomab tiuxetan radioimmunotherapy as an initial therapy of follicular lymphoma: An international phase II study in patients requiring treatment according to GELF/BNLI criteria. J. Clin. Oncol. 2014, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ibatici, A.; Pica, G.M.; Nati, S.; Vitolo, U.; Botto, B.; Ciochetto, C.; Petrini, M.; Galimberti, S.; Ciabatti, E.; Orciuolo, E.; et al. Safety and efficacy of (90) yttrium-ibritumomab-tiuxetan for untreated follicular lymphoma patients. An Italian cooperative study. Br. J. Haematol. 2014, 164, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, F.; Berkova, Z.; Romaguera, J.E.; Fowler, N.; Fanale, M.A.; Pro, B.; Shah, J.J.; McLaughlin, P.; Sehgal, L.; Selvaraj, V.; et al. 90Y-ibritumomab tiuxetan radiotherapy as first-line therapy for early stage low-grade B-cell lymphomas, including bulky disease. Br. J. Haematol. 2014, 167, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Botto, B.; Rohatiner, A.Z.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.; et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: Updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol. 2013, 31, 1977–1983. [Google Scholar] [CrossRef]
- Illidge, T.M.; McKenzie, H.S.; Mayes, S.; Bates, A.; Davies, A.J.; Pettengell, R.; Stanton, L.; Cozens, K.; Hampson, G.; Dive, C.; et al. Short duration immunochemotherapy followed by radioimmunotherapy consolidation is effective and well tolerated in relapsed follicular lymphoma: 5-year results from a UK National Cancer Research Institute Lymphoma Group study. Br. J. Haematol. 2016, 173, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tsujimura, H.; Masaki, Y.; Iino, M.; Takizawa, J.; Maeda, Y.; Yamamoto, K.; Tamura, S.; Yoshida, A.; Yagi, H.; et al. Consolidation with (90) Yttrium-ibritumomab tiuxetan after bendamustine and rituximab for relapsed follicular lymphoma. Hematol. Oncol. 2021, 39, 51–59. [Google Scholar] [CrossRef]
- Roy, R.; Evens, A.M.; Patton, D.; Gallot, L.; Larson, A.; Rademaker, A.; Cilley, J.; Spies, S.; Variakojis, D.; Gordon, L.I.; et al. Bortezomib may be safely combined with Y-90-ibritumomab tiuxetan in patients with relapsed/refractory follicular non-Hodgkin lymphoma: A phase I trial of combined induction therapy and bortezomib consolidation. Leuk. Lymphoma 2013, 54, 497–502. [Google Scholar] [CrossRef]
- Lossos, I.S.; Fabregas, J.C.; Koru-Sengul, T.; Miao, F.; Goodman, D.; Serafini, A.N.; Hosein, P.J.; Stefanovic, A.; Rosenblatt, J.D.; Hoffman, J.E. Phase II study of (90)Y Ibritumomab tiuxetan (Zevalin) in patients with previously untreated marginal zone lymphoma. Leuk. Lymphoma 2015, 56, 1750–1755. [Google Scholar] [CrossRef]
- Vanazzi, A.; Grana, C.; Crosta, C.; Pruneri, G.; Rizzo, S.; Radice, D.; Pinto, A.; Calabrese, L.; Paganelli, G.; Martinelli, G. Efficacy of (9)(0)Yttrium-ibritumomab tiuxetan in relapsed/refractory extranodal marginal-zone lymphoma. Hematol. Oncol. 2014, 32, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hong, F.; Li, H.; Gordon, L.I.; Gascoyne, R.D.; Paietta, E.M.; Advani, R.H.; Forero-Torres, A.; Horning, S.J.; Kahl, B.S. Mantle cell lymphoma initial therapy with abbreviated R-CHOP followed by (90)Y-ibritumomab tiuxetan: 10-year follow-up of the phase 2 ECOG-ACRIN study E1499. Leukemia 2017, 31, 517–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.R.; Li, H.; Gordon, L.; Gascoyne, R.D.; Paietta, E.; Forero-Torres, A.; Kahl, B.S.; Advani, R.; Hong, F.; Horning, S.J. Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium-90-ibritumomab tiuxetan in untreated mantle-cell lymphoma: Eastern Cooperative Oncology Group Study E1499. J. Clin. Oncol. 2012, 30, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, W.; Gruszka, A.M.; Sowa Staszczak, A.; Dlugosz-Danecka, M.; Szostek, M.; Zimowska-Curylo, D.; Giza, A.; Krawczyk, K.; Jakobczyk, M.; Hubalewska-Dydejczyk, A.; et al. Consolidation with (90)Y ibritumomab tiuxetan radioimmunotherapy in mantle cell lymphoma patients ineligible for high dose therapy: Results of the phase II multicentre Polish Lymphoma Research Group trial, after 8-year long follow-up. Leuk. Lymphoma 2019, 60, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.D.; Branger, G.; Klaeser, B.; Taleghani, B.M.; Novak, U.; Banz, Y.; Mueller, B.U.; Pabst, T. Zevalin and BEAM (Z-BEAM) versus rituximab and BEAM (R-BEAM) conditioning chemotherapy prior to autologous stem cell transplantation in patients with mantle cell lymphoma. Hematol. Oncol. 2016, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, A.; Laurell, A.; Jerkeman, M.; Gronbaek, K.; Elonen, E.; Raty, R.; Pedersen, L.B.; Loft, A.; Bogsrud, T.V.; Kimby, E.; et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood 2014, 123, 2953–2959. [Google Scholar] [CrossRef]
- Hohloch, K.; Windemuth-Kieselbach, C.; Zinzani, P.L.; Cacchione, R.; Jurczak, W.; Suh, C.; Trumper, L.; Scholz, C.W. Radioimmunotherapy for mantle cell lymphoma: 5-year follow-up of 90 patients from the international RIT registry. Ann. Hematol. 2020, 99, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaven, A.W.; Shea, T.C.; Moore, D.T.; Feldman, T.; Ivanova, A.; Ferraro, M.; Ford, P.; Smith, J.; Goy, A. A phase I study evaluating ibritumomab tiuxetan (Zevalin(R)) in combination with bortezomib (Velcade(R)) in relapsed/refractory mantle cell and low grade B-cell non-Hodgkin lymphoma. Leuk. Lymphoma 2012, 53, 254–258. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, S.E.; Kim, S.H.; Sohn, H.S.; Jung, S.E.; Park, G.; Choi, B.O.; Lee, S.N.; Yang, S.W.; Han, K.; et al. Clinical outcomes of post-remission therapy using (90)yttrium ibritumomab tiuxetan (Zevalin(R)) for high-risk patients with diffuse large B-cell lymphoma. Ann. Hematol. 2011, 90, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Lugtenburg, P.J.; Zijlstra, J.M.; Doorduijn, J.K.; Bohmer, L.H.; Hoogendoorn, M.; Berenschot, H.W.; Beeker, A.; van der Burg-de Graauw, N.C.; Schouten, H.C.; Bilgin, Y.M.; et al. Rituximab-PECC induction followed by (90) Y-ibritumomab tiuxetan consolidation in relapsed or refractory DLBCL patients who are ineligible for or have failed ASCT: Results from a phase II HOVON study. Br. J. Haematol. 2019, 187, 347–355. [Google Scholar] [CrossRef]
- Briones, J.; Novelli, S.; Garcia-Marco, J.A.; Tomas, J.F.; Bernal, T.; Grande, C.; Canales, M.A.; Torres, A.; Moraleda, J.M.; Panizo, C.; et al. Autologous stem cell transplantation after conditioning with yttrium-90 ibritumomab tiuxetan plus BEAM in refractory non-Hodgkin diffuse large B-cell lymphoma: Results of a prospective, multicenter, phase II clinical trial. Haematologica 2014, 99, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, M.; Wondergem, M.J.; Palmer, J.M.; Shimoni, A.; Hasenkamp, J.; Tsai, N.C.; Simpson, J.; Nademanee, A.; Raubitschek, A.; Forman, S.J.; et al. Autologous transplantation for transformed non-Hodgkin lymphoma using an yttrium-90 ibritumomab tiuxetan conditioning regimen. Biol. Blood Marrow Transplant. 2014, 20, 2072–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koechli, V.; Klaeser, B.; Banz, Y.; Mueller, B.U.; Pabst, T. Consolidation of first remission using radioimmunotherapy with yttrium-90-ibritumomab-tiuxetan in adult patients with Burkitt lymphoma. Leuk. Res. 2015, 39, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Bethge, W.A.; von Harsdorf, S.; Bornhauser, M.; Federmann, B.; Stelljes, M.; Trenschel, R.; Baurmann, H.; Dittmann, H.; Faul, C.; Vogel, W.; et al. Dose-escalated radioimmunotherapy as part of reduced intensity conditioning for allogeneic transplantation in patients with advanced high-grade non-Hodgkin lymphoma. Bone Marrow Transplant. 2012, 47, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrero, M.; Martin, A.; Briones, J.; Gayoso, J.; Jarque, I.; Lopez, J.; Grande, C.; Heras, I.; Arranz, R.; Bernal, T.; et al. Phase II Study of Yttrium-90-Ibritumomab Tiuxetan as Part of Reduced-Intensity Conditioning (with Melphalan, Fludarabine +/− Thiotepa) for Allogeneic Transplantation in Relapsed or Refractory Aggressive B Cell Lymphoma: A GELTAMO Trial. Biol. Blood Marrow Transplant. 2017, 23, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.Y.; Palmer, J.; Nademanee, A.P.; Chen, R.; Popplewell, L.L.; Tsai, N.C.; Sanchez, J.F.; Simpson, J.; Spielberger, R.; Yamauchi, D.; et al. Phase II Study of Yttrium-90 Ibritumomab Tiuxetan Plus High-Dose BCNU, Etoposide, Cytarabine, and Melphalan for Non-Hodgkin Lymphoma: The Role of Histology. Biol. Blood Marrow Transplant. 2017, 23, 922–929. [Google Scholar] [CrossRef] [Green Version]
- Gopal, A.K.; Guthrie, K.A.; Rajendran, J.; Pagel, J.M.; Oliveira, G.; Maloney, D.G.; Matesan, M.C.; Storb, R.F.; Press, O.W. (9)(0)Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood 2011, 118, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Cassaday, R.D.; Storer, B.E.; Sorror, M.L.; Sandmaier, B.M.; Guthrie, K.A.; Maloney, D.G.; Rajendran, J.G.; Pagel, J.M.; Flowers, M.E.; Green, D.J.; et al. Long-term outcomes of patients with persistent indolent B cell malignancies undergoing nonmyeloablative allogeneic transplantation. Biol. Blood Marrow Transplant. 2015, 21, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Ria, R.; Musto, P.; Reale, A.; Guariglia, R.; Iodice, G.; Dammacco, F.; Vacca, A. 90Y-ibritumomab tiuxetan as consolidation therapy after autologous stem cell transplantation in aggressive non-Hodgkin lymphoma. J. Nucl. Med. 2011, 52, 891–895. [Google Scholar] [CrossRef] [Green Version]
- Witzig, T.E.; Wiseman, G.A.; Maurer, M.J.; Habermann, T.M.; Micallef, I.N.; Nowakowski, G.S.; Ansell, S.M.; Colgan, J.P.; Inwards, D.J.; Porrata, L.F.; et al. A phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and 90 yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-Hodgkin lymphoma. Am. J. Hematol. 2013, 88, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puvvada, S.D.; Guillen-Rodriguez, J.M.; Yan, J.; Inclan, L.; Heard, K.; Rivera, X.I.; Anwer, F.; Mahadevan, D.; Schatz, J.H.; Persky, D.O. Yttrium-90-Ibritumomab Tiuxetan (Zevalin(R)) Radioimmunotherapy after Cytoreduction with ESHAP Chemotherapy in Patients with Relapsed Follicular Non-Hodgkin Lymphoma: Final Results of a Phase II Study. Oncology 2018, 94, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Vaklavas, C.; Meredith, R.F.; Shen, S.; Knox, S.J.; Micallef, I.N.; Shah, J.J.; LoBuglio, A.F.; Forero-Torres, A. Phase I study of a modified regimen of (9)(0)Yttrium-ibritumomab tiuxetan for relapsed or refractory follicular or transformed CD20+ non-Hodgkin lymphoma. Cancer Biother. Radiopharm. 2013, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; D’Souza, A.; Gertz, M.A.; Laumann, K.; Wiseman, G.; Lacy, M.Q.; LaPlant, B.; Buadi, F.; Hayman, S.R.; Kumar, S.K.; et al. A phase 1 trial of (90)Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma. Bone Marrow Transplant. 2017, 52, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, A.D.; Macdonald, W.B.; Turner, J.H. Phase II study of first-line (131)I-rituximab radioimmunotherapy in follicular non-Hodgkin lymphoma and prognostic (18)F-fluorodeoxyglucose positron emission tomography. Leuk. Lymphoma 2015, 56, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.Y.; Schwarz, K.; Schreiber, S.; Schmidt, B.; Wester, H.J.; Schwaiger, M.; Peschel, C.; von Schilling, C.; Scheidhauer, K.; Keller, U. Myeloablative anti-CD20 radioimmunotherapy +/− high-dose chemotherapy followed by autologous stem cell support for relapsed/refractory B-cell lymphoma results in excellent long-term survival. Oncotarget 2013, 4, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Lee, S.S.; Byun, B.H.; Kim, K.M.; Lim, I.; Choi, C.W.; Suh, C.; Kim, W.S.; Nam, S.H.; Lee, S.I.; et al. Repeated radioimmunotherapy with 131I-rituximab for patients with low-grade and aggressive relapsed or refractory B cell non-Hodgkin lymphoma. Cancer Chemother. Pharmacol. 2013, 71, 945–953. [Google Scholar] [CrossRef]
- Lee, I.; Byun, B.H.; Lim, I.; Kim, B.I.; Choi, C.W.; Kim, K.M.; Shin, D.Y.; Kang, H.J.; Lim, S.M. Comparisons of (131)I-rituximab treatment responses in patients with aggressive lymphoma and indolent lymphoma. Ann. Nucl. Med. 2019, 33, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Forrer, F.; Oechslin-Oberholzer, C.; Campana, B.; Herrmann, R.; Maecke, H.R.; Mueller-Brand, J.; Lohri, A. Radioimmunotherapy with 177Lu-DOTA-rituximab: Final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J. Nucl. Med. 2013, 54, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.P.; Singla, S.; Thakral, P.; Ballal, S.; Bal, C. Dosimetric analysis of 177Lu-DOTA-rituximab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Nucl. Med. Commun. 2016, 37, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Thakral, P.; Singla, S.; Vashist, A.; Yadav, M.P.; Gupta, S.K.; Tyagi, J.S.; Sharma, A.; Bal, C.S.; Snehlata, E.Y.; Malhotra, A. Preliminary Experience with Yttrium-90-labelled Rituximab (Chimeric Anti CD-20 Antibody) in Patients with Relapsed and Refractory B Cell Non-Hodgkins Lymphoma. Curr. Radiopharm. 2016, 9, 160–168. [Google Scholar] [CrossRef]
- Press, O.W.; Unger, J.M.; Rimsza, L.M.; Friedberg, J.W.; LeBlanc, M.; Czuczman, M.S.; Kaminski, M.; Braziel, R.M.; Spier, C.; Gopal, A.K.; et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J. Clin. Oncol. 2013, 31, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadman, M.; Li, H.; Rimsza, L.; Leonard, J.P.; Kaminski, M.S.; Braziel, R.M.; Spier, C.M.; Gopal, A.K.; Maloney, D.G.; Cheson, B.D.; et al. Continued Excellent Outcomes in Previously Untreated Patients With Follicular Lymphoma After Treatment With CHOP Plus Rituximab or CHOP Plus (131)I-Tositumomab: Long-Term Follow-Up of Phase III Randomized Study SWOG-S0016. J. Clin. Oncol. 2018, 36, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quackenbush, R.C.; Horner, T.J.; Williams, V.C.; Giampietro, P.; Lin, T.S. Patients with relapsed follicular lymphoma treated with rituximab versus tositumomab and iodine I-131 tositumomab. Leuk. Lymphoma 2015, 56, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Olney, H.J.; Freeman, M.A.; Stewart, D.A.; Mangel, J.E.; White, D.J.; Elia-Pacitti, J.O. Prolonged progression-free survival and preserved quality of life in the Canadian prospective study of tositumomab and iodine(131)-tositumomab for previously treated, rituximab-exposed, indolent non-Hodgkin lymphoma. Leuk. Lymphoma 2014, 55, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.W.; Unger, J.M.; Burack, W.R.; Gopal, A.K.; Raju, R.N.; Nademanee, A.P.; Kaminski, M.S.; Li, H.; Press, O.W.; Miller, T.P.; et al. R-CHOP with iodine-131 tositumomab consolidation for advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0433. Br. J. Haematol. 2014, 166, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Hudson, K.E.; Rizzieri, D.; Thomas, S.M.; LeBlanc, T.W.; Powell, Z.; Diehl, L.; Moore, J.O.; DeCastro, C.; Beaven, A.W. Dose-intense chemoimmunotherapy plus radioimmunotherapy in high-risk diffuse large B-cell lymphoma and mantle cell lymphoma: A phase II study. Br. J. Haematol. 2019, 184, 647–650. [Google Scholar] [CrossRef] [Green Version]
- Vose, J.M.; Carter, S.; Burns, L.J.; Ayala, E.; Press, O.W.; Moskowitz, C.H.; Stadtmauer, E.A.; Mineshi, S.; Ambinder, R.; Fenske, T.; et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: Results from the BMT CTN 0401 trial. J. Clin. Oncol. 2013, 31, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Chow, V.A.; Rajendran, J.G.; Fisher, D.R.; Appelbaum, F.R.; Cassaday, R.D.; Martin, P.S.; Holmberg, L.A.; Gooley, T.A.; Stevenson, P.A.; Pagel, J.M.; et al. A phase II trial evaluating the efficacy of high-dose Radioiodinated Tositumomab (Anti-CD20) antibody, etoposide and cyclophosphamide followed by autologous transplantation, for high-risk relapsed or refractory non-hodgkin lymphoma. Am. J. Hematol. 2020, 95, 775–783. [Google Scholar] [CrossRef]
- Gopal, A.K.; Gooley, T.A.; Rajendran, J.G.; Pagel, J.M.; Fisher, D.R.; Maloney, D.G.; Appelbaum, F.R.; Cassaday, R.D.; Shields, A.; Press, O.W. Myeloablative I-131-tositumomab with escalating doses of fludarabine and autologous hematopoietic transplantation for adults age >/= 60 years with B cell lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewaraja, Y.K.; Schipper, M.J.; Shen, J.; Smith, L.B.; Murgic, J.; Savas, H.; Youssef, E.; Regan, D.; Wilderman, S.J.; Roberson, P.L.; et al. Tumor-Absorbed Dose Predicts Progression-Free Survival Following (131)I-Tositumomab Radioimmunotherapy. J. Nucl. Med. 2014, 55, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstrom, R.L.; Ruan, J.; Christos, P.J.; Martin, P.; Lebovic, D.; Osborne, J.; Goldsmith, S.; Greenberg, J.; Furman, R.R.; Avram, A.; et al. Phase 1 study of radiosensitization using bortezomib in patients with relapsed non-Hodgkin lymphoma receiving radioimmunotherapy with 131I-tositumomab. Leuk. Lymphoma 2015, 56, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacene, H.; Crandall, J.; Kasamon, Y.L.; Ambinder, R.F.; Piantadosi, S.; Serena, D.; Kasecamp, W.; Wahl, R.L. Initial Experience with Tositumomab and I-131-Labeled Tositumomab for Treatment of Relapsed/Refractory Hodgkin Lymphoma. Mol. Imaging Biol. 2017, 19, 429–436. [Google Scholar] [CrossRef]
- Chevallier, P.; Eugene, T.; Robillard, N.; Isnard, F.; Nicolini, F.; Escoffre-Barbe, M.; Huguet, F.; Hunault, M.; Marcais, A.; Gaschet, J.; et al. (90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: A phase 1 dose-escalation study. Lancet Haematol. 2015, 2, e108–e117. [Google Scholar] [CrossRef]
- Kraeber-Bodere, F.; Pallardy, A.; Maisonneuve, H.; Campion, L.; Moreau, A.; Soubeyran, I.; Le Gouill, S.; Tournilhac, O.; Daguindau, E.; Jardel, H.; et al. Consolidation anti-CD22 fractionated radioimmunotherapy with (90)Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: A prospective, single group, phase 2 trial. Lancet Haematol. 2017, 4, e35–e45. [Google Scholar] [CrossRef]
- Witzig, T.E.; Tomblyn, M.B.; Misleh, J.G.; Kio, E.A.; Sharkey, R.M.; Wegener, W.A.; Goldenberg, D.M. Anti-CD22 90Y-epratuzumab tetraxetan combined with anti-CD20 veltuzumab: A phase I study in patients with relapsed/refractory, aggressive non-Hodgkin lymphoma. Haematologica 2014, 99, 1738–1745. [Google Scholar] [CrossRef] [Green Version]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Strumpf, A.; Schetelig, J.; Wunderlich, G.; Ehninger, G.; Kotzerke, J.; Bornhauser, M. Reduced-Intensity Conditioning Combined with (188)Rhenium Radioimmunotherapy before Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: The Role of In Vivo T Cell Depletion. Biol. Blood Marrow Transplant. 2015, 21, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Schulz, A.S.; Glatting, G.; Hoenig, M.; Schuetz, C.; Gatz, S.A.; Grewendorf, S.; Sparber-Sauer, M.; Muche, R.; Blumstein, N.; Kropshofer, G.; et al. Radioimmunotherapy-based conditioning for hematopoietic cell transplantation in children with malignant and nonmalignant diseases. Blood 2011, 117, 4642–4650. [Google Scholar] [CrossRef] [Green Version]
- Kramer, K.; Pandit-Taskar, N.; Zanzonico, P.; Wolden, S.L.; Humm, J.L.; DeSelm, C.; Souweidane, M.M.; Lewis, J.S.; Cheung, N.-K.V. Low incidence of radionecrosis in children treated with conventional radiation therapy and intrathecal radioimmunotherapy. J. Neuro-Oncol. 2015, 123, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef] [Green Version]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhusari, P.; Vatsa, R.; Singh, G.; Parmar, M.; Bal, A.; Dhawan, D.K.; Mittal, B.R.; Shukla, J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 2017, 140, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Pandit-Taskar, N.; Humm, J.L.; Zanzonico, P.B.; Haque, S.; Dunkel, I.J.; Wolden, S.L.; Donzelli, M.; Goldman, D.A.; Lewis, J.S.; et al. A phase II study of radioimmunotherapy with intraventricular (131) I-3F8 for medulloblastoma. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef]
- Herbertson, R.A.; Tebbutt, N.C.; Lee, F.T.; Gill, S.; Chappell, B.; Cavicchiolo, T.; Saunder, T.; O’Keefe, G.J.; Poon, A.; Lee, S.T.; et al. Targeted chemoradiation in metastatic colorectal cancer: A phase I trial of 131I-huA33 with concurrent capecitabine. J. Nucl. Med. 2014, 55, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Cahan, B.; Leong, L.; Wagman, L.; Yamauchi, D.; Shibata, S.; Wilzcynski, S.; Williams, L.E.; Yazaki, P.; Colcher, D.; Frankel, P.; et al. Phase I/II Trial of Anticarcinoembryonic Antigen Radioimmunotherapy, Gemcitabine, and Hepatic Arterial Infusion of Fluorodeoxyuridine Postresection of Liver Metastasis for Colorectal Carcinoma. Cancer Biother. Radiopharm. 2017, 32, 258–265. [Google Scholar] [CrossRef]
- Sahlmann, C.O.; Homayounfar, K.; Niessner, M.; Dyczkowski, J.; Conradi, L.C.; Braulke, F.; Meller, B.; Beissbarth, T.; Ghadimi, B.M.; Meller, J.; et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 2017, 123, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of patient survival in a Phase I trial of systemic targeted alpha-therapy for metastatic melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Cohen, S.J.; Pennington, K.L.; Zuckier, L.S.; Hauke, R.J.; Horne, H.; Wegener, W.A.; Teoh, N.; Gold, D.V.; Sharkey, R.M.; et al. Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: A phase I single-dose escalation trial. Clin. Cancer Res. 2011, 17, 4091–4100. [Google Scholar] [CrossRef] [Green Version]
- Ocean, A.J.; Pennington, K.L.; Guarino, M.J.; Sheikh, A.; Bekaii-Saab, T.; Serafini, A.N.; Lee, D.; Sung, M.W.; Gulec, S.A.; Goldsmith, S.J.; et al. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer 2012, 118, 5497–5506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picozzi, V.J.; Ramanathan, R.K.; Lowery, M.A.; Ocean, A.J.; Mitchel, E.P.; O’Neil, B.H.; Guarino, M.J.; Conkling, P.R.; Cohen, S.J.; Bahary, N.; et al. (90)Y-clivatuzumab tetraxetan with or without low-dose gemcitabine: A phase Ib study in patients with metastatic pancreatic cancer after two or more prior therapies. Eur. J. Cancer 2015, 51, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177–Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, S.T.; Vallabhajosula, S.; Christos, P.J.; Jhanwar, Y.S.; Batra, J.S.; Lam, L.; Osborne, J.; Beltran, H.; Molina, A.M.; Goldsmith, S.J.; et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177) Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 2019, 125, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.S.; Niaz, M.J.; Whang, Y.E.; Sheikh, A.; Thomas, C.; Christos, P.; Vallabhajosula, S.; Jhanwar, Y.S.; Molina, A.M.; Nanus, D.M.; et al. Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177)Lu-J591) for metastatic castration-resistant prostate cancer. Urol. Oncol. 2020, 38, 848.e9–848.e16. [Google Scholar] [CrossRef] [PubMed]
- Niaz, M.J.; Batra, J.S.; Walsh, R.D.; Ramirez-Fort, M.K.; Vallabhajosula, S.; Jhanwar, Y.S.; Molina, A.M.; Nanus, D.M.; Osborne, J.R.; Bander, N.H.; et al. Pilot Study of Hyperfractionated Dosing of Lutetium-177-Labeled Antiprostate-Specific Membrane Antigen Monoclonal Antibody J591 ((177) Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. Oncologist 2020, 25, 477-e895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillebroer, A.B.; Boerman, O.C.; Desar, I.M.; Boers-Sonderen, M.J.; van Herpen, C.M.; Langenhuijsen, J.F.; Smith-Jones, P.M.; Oosterwijk, E.; Oyen, W.J.; Mulders, P.F. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 2013, 64, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Muselaers, C.H.; Boers-Sonderen, M.J.; van Oostenbrugge, T.J.; Boerman, O.C.; Desar, I.M.; Stillebroer, A.B.; Mulder, S.F.; van Herpen, C.M.; Langenhuijsen, J.F.; Oosterwijk, E.; et al. Phase 2 Study of Lutetium 177-Labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2016, 69, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Su, Z.; Zhang, W.; Luo, M.; Wang, H.; Huang, L. A randomized study comparing the effectiveness of microwave ablation radioimmunotherapy and postoperative adjuvant chemoradiation in the treatment of non-small cell lung cancer. J. BUON 2016, 21, 326–332. [Google Scholar]
- Giraudet, A.L.; Cassier, P.A.; Iwao-Fukukawa, C.; Garin, G.; Badel, J.N.; Kryza, D.; Chabaud, S.; Gilles-Afchain, L.; Clapisson, G.; Desuzinges, C.; et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer 2018, 18, 646. [Google Scholar] [CrossRef]
- Modak, S.; Zanzonico, P.; Grkovski, M.; Slotkin, E.K.; Carrasquillo, J.A.; Lyashchenko, S.K.; Lewis, J.S.; Cheung, I.Y.; Heaton, T.; LaQuaglia, M.P.; et al. B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J. Clin. Oncol. 2020, 38, 4283–4291. [Google Scholar] [CrossRef]
- Lu, G.; Nishio, N.; van den Berg, N.S.; Martin, B.A.; Fakurnejad, S.; van Keulen, S.; Colevas, A.D.; Thurber, G.M.; Rosenthal, E.L. Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat. Commun. 2020, 11, 5667. [Google Scholar] [CrossRef]
- White, J.M.; Escorcia, F.E.; Viola, N.T. Perspectives on metals-based radioimmunotherapy (RIT): Moving forward. Theranostics 2021, 11, 6293–6314. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef]
- Rondon, A.; Mahri, S.; Morales-Yanez, F.; Dumoulin, M.; Vanbever, R. Protein Engineering Strategies for Improved Pharmacokinetics. Adv. Funct. Mater. 2021, 31, 2101633. [Google Scholar] [CrossRef]
- Kholodenko, R.V.; Kalinovsky, D.V.; Doronin, I.I.; Ponomarev, E.D.; Kholodenko, I.V. Antibody Fragments as Potential Biopharmaceuticals for Cancer Therapy: Success and Limitations. Curr. Med. Chem. 2019, 26, 396–426. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.L. Antibody fragments: Hope and hype. MAbs 2010, 2, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009, 27, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.L.; Napier, M.P.; King, D.J.; Pedley, R.B.; Chaplin, L.C.; Weir, N.; Skelton, L.; Green, A.J.; Hope-Stone, L.D.; Yarranton, G.T.; et al. Tumour targeting of humanised cross-linked divalent-Fab′ antibody fragments: A clinical phase I/II study. Br. J. Cancer 2002, 86, 1401–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeer, A.W.P.; Norde, W. The Thermal Stability of Immunoglobulin: Unfolding and Aggregation of a Multi-Domain Protein. Biophys. J. 2000, 78, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Aloj, L.; D’Ambrosio, L.; Aurilio, M.; Morisco, A.; Frigeri, F.; Caraco, C.; Di Gennaro, F.; Capobianco, G.; Giovannoni, L.; Menssen, H.D.; et al. Radioimmunotherapy with Tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 867–877. [Google Scholar] [CrossRef]
- Gonzalez, G.P.; Garcia, I.G.; Gonzalez, J.G.; Sanchez, L.P.; Mirabal, M.V.; Marin, C.C.; Ruiz, F.L.; Iglesias, E.G.; de Queralta, R.L.; Toirac, R.R.; et al. Phase I clinical trial of the (131)I-labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment. Cancer Biother. Radiopharm. 2011, 26, 353–363. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.F.; Ge, N.J.; Shen, S.Q.; Liang, J.; Wang, Y.; Zhou, W.P.; Shen, F.; Wu, M.C. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: Interim safety and survival data from 110 patients. Cancer Biother. Radiopharm. 2010, 25, 657–663. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.F.; Ge, N.J.; Shen, S.Q.; Liang, J.; Wang, Y.; Zhou, W.P.; Shen, F.; Wu, M.C. Hepatic artery injection of (1)(3)(1)I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: A prospective nonrandomized study. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, J.X.; Ren, J.Z.; Zhang, W.J.; Han, X.W. Clinical value of iodine [131I] metuximab infusion combined with TACE for treatment of patients with post-intervention relapse of mid or advanced stage hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2013, 21, 728–733. [Google Scholar] [CrossRef]
- Bian, H.; Zheng, J.S.; Nan, G.; Li, R.; Chen, C.; Hu, C.X.; Zhang, Y.; Sun, B.; Wang, X.L.; Cui, S.C.; et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xing, J.; Yang, Y.; Liu, J.; Wang, W.; Xia, Y.; Yan, Z.; Wang, K.; Wu, D.; Wu, L.; et al. Adjuvant (131)I-metuximab for hepatocellular carcinoma after liver resection: A randomised, controlled, multicentre, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2020, 5, 548–560. [Google Scholar] [CrossRef]
- Breitz, H.B.; Tyler, A.; Bjorn, M.J.; Lesley, T.; Weiden, P.L. Clinical experience with Tc-99m nofetumomab merpentan (Verluma) radioimmunoscintigraphy. Clin. Nucl. Med. 1997, 22, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.M.; Gnanasegaran, G.; Buscombe, J.R.; Hilson, A.J. Technetium-99m-labelled sulesomab (LeukoScan) in the evaluation of soft tissue infections. Med. Princ. Pract. 2008, 17, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Yates, S.; Smith, S.L.; Dudley, S., Jr.; Schmidt, J.O.; Shirazi, F.M. Scorpion Stings and Antivenom Use in Arizona. Am. J. Med. 2021, 134, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Cocchio, C.; Johnson, J.; Clifton, S. Review of North American pit viper antivenoms. Am. J. Health Syst. Pharm 2020, 77, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.N.; Mi, L.; Xu, J.; Song, F.; Zhang, Q.; Zhang, Z.; Xing, J.L.; Bian, H.J.; Jiang, J.L.; Wang, X.H.; et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 435–444. [Google Scholar] [CrossRef]
- Xu, J.; Shen, Z.-Y.; Chen, X.-G.; Zhang, Q.; Bian, H.-J.; Zhu, P.; Xu, H.-Y.; Song, F.; Yang, X.-M.; Mi, L.; et al. A randomized controlled trial of licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007, 45, 269–276. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.-Y.; Zhang, Q.; Song, F.; Jiang, J.-L.; Yang, X.-M.; Mi, L.; Wen, N.; Tian, R.; Wang, L.; et al. HAb18G/CD147 Functions in Invasion and Metastasis of Hepatocellular Carcinoma. Mol. Cancer Res. 2007, 5, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yuan, Y.; Jiang, X. Antibody and antibody fragments for cancer immunotherapy. J. Control. Release 2020, 328, 395–406. [Google Scholar] [CrossRef]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, A. Brolucizumab: First Approval. Drugs 2019, 79, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Xavier, C.; Muyldermans, S.; Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin. Drug Deliv. 2016, 13, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Karacay, H.; Cardillo, T.M.; Chang, C.H.; McBride, W.J.; Rossi, E.A.; Horak, I.D.; Goldenberg, D.M. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin. Cancer Res. 2005, 11, 7109s–7121s. [Google Scholar] [CrossRef] [Green Version]
- Rondon, A.; Degoul, F. Antibody Pretargeting Based on Bioorthogonal Click Chemistry for Cancer Imaging and Targeted Radionuclide Therapy. Bioconjug Chem. 2020, 31, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Shim, H. Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J.; Kim, E.S. Emicizumab-kxwh: First Global Approval. Drugs 2018, 78, 269–274. [Google Scholar] [CrossRef]
- Salaun, P.Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: Biomarker response and survival improvement. J. Nucl. Med. 2012, 53, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.; Franssen, G.M.; McBride, W.J.; Chang, C.H.; Rossi, E.A.; van der Graaf, W.T.; et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br. J. Cancer 2013, 109, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodet-Milin, C.; Ferrer, L.; Rauscher, A.; Masson, D.; Rbah-Vidal, L.; Faivre-Chauvet, A.; Cerato, E.; Rousseau, C.; Hureaux, J.; Couturier, O.; et al. Pharmacokinetics and Dosimetry Studies for Optimization of Pretargeted Radioimmunotherapy in CEA-Expressing Advanced Lung Cancer Patients. Front. Med. 2015, 2, 84. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.A.; Goldenberg, D.M.; Cardillo, T.M.; McBride, W.J.; Sharkey, R.M.; Chang, C.H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. USA 2006, 103, 6841–6846. [Google Scholar] [CrossRef] [Green Version]




| Target/ | Isotope | Cancer Type 1 | n2 | Type of Studies | Line of Treatment | [Ref] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vector | I | I/II | II | III | Others 3 | 1st Line | Conditioning | Consolidation | R/R 4 | ||||
| Ibritumomab tiuxetan | Y-90 | FL | 12 | 1 | 1 | 9 | 1 | − | 4 | 1 | 6 | 1 | [10,11,12,13,14,15,16,17,18,19,20,21] |
| MZL | 4 | − | − | 3 | − | 1 (P) | 2 | − | 1 | 1 | [13,17,22,23] | ||
| MCL | 7 | 1 | − | 4 | − | 1(R)/1(P) | − | 1 | 5 | 1 | [24,25,26,27,28,29,30] | ||
| DLBCL | 4 | − | − | 3 | − | 1(R) | − | 2 | 2 | − | [31,32,33,34] | ||
| Burkitt Lymphoma | 1 | − | − | − | − | 1(R) | − | − | 1 | − | [35] | ||
| B-NHL | 9 | 2 | − | 5 | − | 1(R)/1(CS) | − | 5 | 1 | 3 | [36,37,38,39,40,41,42,43,44] | ||
| MM | 1 | 1 | − | − | − | − | − | 1 | − | − | [45] | ||
| Rituximab | I-131 | FL | 1 | − | − | 1 | − | − | 1 | − | − | − | [46] |
| B-NHL | 3 | − | 1 | 1 | − | 1(R) | − | 1 | − | 2 | [47,48,49] | ||
| Lu-177 | Low-grade B-NHL | 1 | − | 1 | − | − | − | − | − | − | 1 | [50] | |
| B-NHL | 1 | − | − | − | − | 1(P) | − | − | − | 1 | [51] | ||
| Y-90 | B-NHL | 1 | − | − | − | − | 1(CS) | − | − | − | 1 | [52] | |
| Tositumomab | I-131 | FL | 2 | − | − | − | 2 | − | − | − | 2 | − | [53,54] |
| Low-grade B-NHL | 2 | − | − | 1 | 1 | − | − | − | − | 2 | [55,56] | ||
| DLBCL | 3 | − | − | 2 | 1 | − | − | 1 | 2 | − | [57,58,59] | ||
| B-NHL | 2 | 2 | − | 1 | − | 1(CS) | 2 | − | 2 | [60,61,62,63] | |||
| HL | 1 | 1 | − | − | − | − | − | − | − | 1 | [64] | ||
| Target/ Vector | Isotope | Cancer Type 1 | Phase | n 2 | Line of Treatment 3 | Association 4 (+/−) | SCT (Auto/Allo/−) | Cold mAb (+/−) | Fraction-Ation * (+/−) | PFS Median (Months) or X Years PF 5 (%) | OS Median (Months) or X Years OS (%) | ORR (%) | CR (%) | NCT (or eq.), [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD22 | ||||||||||||||
| Epratuzumab tetraxetan | Y-90 | ALL | I | 17 | R/R | − | − | + | − | 6.1 | 3.6 | − | − | NCT01354457, [65] |
| DLBCL | II | 71 | Consolidation | + (C) | − | + (anti-CD20 mAb) | − | NR 2 y: 82% | NR 2 y: 89% | − | 77 | NCT00906841, [66] | ||
| B-NHL | I | 18 | R/R | − | − | + (anti-CD20 mAb) | − | 6.2 | − | 53 | 18 | - [67] | ||
| CD33 | ||||||||||||||
| Lintuzumab | Bi-213 | AML | I/II | 31 | 1st line or R/R | + (C) | − | − | + | − | 4.6 | 19 | 10 | - [68] |
| CD66 | ||||||||||||||
| Anti-CD66 | Re-188 | AML | II | 58 | Conditioning | + (C+I) | Allo | − | − | 2 y DFS: 38% | 2 y: 38% | − | − | - [69] |
| BW250-183 | Y-90 | Pediatric malignant (Mal.) and non-maligant (Non-Mal.) hemopathies | II | 30 | Conditioning | + (C) | Auto Allo | − | − | Mal.: 12 Non-Mal.: NR | Mal.: NR Non-Mal.: NR | − | − | - [70] |
| Target | Vector | n1 | Phase | Isotope | Application 2 | Year of Publication | NCT or eq. | Ref |
|---|---|---|---|---|---|---|---|---|
| Non-solid cancer | ||||||||
| Tenascin C | F16SIP F(ab′)2 | 8 | I/II | I-131 | HL | 2014 | EudraCT2007-007259-15 | [101] |
| Solid cancers | ||||||||
| CEA | CIGB-M3 ScFv | 17 | I | I-131 | CRC * | 2011 | – | [102] |
| CD147 | HAb18 metuximab F(ab′)2 (Licartin®) | 110 | III | I-131 | HCC * | 2010 | NCT00829465 | [103] |
| 68 | P | I-131 | HCC * | 2012 | ChiCTR-TRC-08000250 | [104] | ||
| 60 | II | I-131 | HCC * | 2013 | – | [105] | ||
| 127 | III | I-131 | HCC * | 2014 | ChiCTR-TRC-10000837 | [106] | ||
| 156 | II | I-131 | HCC * | 2020 | NCT00819650 | [107] | ||
| Target | Antibody | Vector | n | Phase | Isotope | Application 1 | Year of Publication | NCT | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CEA | hMN14 × m734 F(ab′)2 | Di-DTPA | 42 | II | I-131 | MTC | 2012 | NCT00467506 | [125] |
| 63 | II | I-131 | mCRC | 2016 | - | [78] | |||
| TF2 | IMP-288 | 21 | I | Lu-177 | mCRC | 2013 | NCT00860860 | [126] | |
| 18 | I/II | Lu-177 | SCLC and NSCLC | 2015 | NCT01221675, EudraCT 200800603096 | [127] |
| NCT | Starting Date | Phase | Target | Vector | Isotope | Application 1 | Administration Route 2 | Association 3 (+/−) | Cold mAb (+/−) |
|---|---|---|---|---|---|---|---|---|---|
| Non-solid tumors | |||||||||
| NCT01796171 | 2012 | I/II | CD37 | Betalutin | Lu-177 | NHL/FL | I.V | - | + |
| NCT02320292 | 2015 | III | CD20 | Ibritumomab tiuxetan | Y-90 | FL | I.V | - | + |
| NCT04082286 | 2016 | I | CD66 | Anti-CD66 | Y-90 | AL/AML | I.V | + (T) | - |
| NCT03128034 | 2017 | I/II | CD45 | BC8-B10 | At-211 | AML | I.V | + (C + R) | - |
| NCT02658968 | 2017 | I | CD37 | Betalutin | Lu-177 | DLBCL | I.V | - | + |
| NCT03806179 | 2018 | I | CD37 | Betalutin | Lu-177 | NHL/FL | I.V | - | + |
| NCT04466475 | 2020 | I | CD38 | OK10-B10 | At-211 | MM | I.V | + (C + T) | |
| NCT04083183 | 2020 | I/II | CD45 | BC8-B10 | At-211 | Non-mal. hemopathies | I.V | + (C + T + R) | + |
| NCT04856215 | 2021 | II | CD66 | Anti-CD66 | Y-90 | Leukemia | I.V. | + (T + R) | - |
| NCT04871607 | 2021 | II | CD25 | Basiliximab | Y-90 | R/R HL | I.V. | + (C + R + T) | + |
| Solid tumors | |||||||||
| NCT02454010 | 2015 | I | CDH3 | FF21101 | Y-90 | Adv. Solid T. | Not. Spec. | - | - |
| ChiCTR-IPR-17011206 | 2017 | III | CD147 | Metuximab * | I-131 | HCC | I.V. | – | – |
| NCT03724747 | 2018 | I | Mesothelin | BAY2315497 | Th-227 | mCRPC | Not Spec. | + (H) | - |
| NCT03507452 | 2018 | I | BAY2287411 | Th-227 | OC, Adv. Solid T. | Not Spec. | - | - | |
| NCT03275402 | 2018 | II/III | B7H3 | Omburtamab | I-131 | CNS meta. | I.C | - | - |
| NCT04022213 | 2019 | II | I-131 | SRCT/PC | + (R) | - | |||
| NCT04644770 | 2020 | I | hK21 | h11B6 | Ac-225 | mCRPC | I.V. | - | - |
| NCT04674722 | 2020 | I | HER2 | NM02 ** | Re-188 | Breast cancer | I.V. | - | - |
| NCT04315246 | 2020 | I/II | B7H3 | Omburtamab | Lu-177 | LP meta. | I.V. | - | - |
| NCT04167618 | 2020 | I/II | Lu-177 | MB | - | - | |||
| NCT04743661 Eudra CT 2020-000670-22 | 2021 | II | I-131 | MB | + (C) | - | |||
| NCT03276572 | 2021 | I | PSMA | J591 | Ac-225 | mCRPC | I.V | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13, 5570. https://doi.org/10.3390/cancers13215570
Rondon A, Rouanet J, Degoul F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers. 2021; 13(21):5570. https://doi.org/10.3390/cancers13215570
Chicago/Turabian StyleRondon, Aurélie, Jacques Rouanet, and Françoise Degoul. 2021. "Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials" Cancers 13, no. 21: 5570. https://doi.org/10.3390/cancers13215570
APA StyleRondon, A., Rouanet, J., & Degoul, F. (2021). Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers, 13(21), 5570. https://doi.org/10.3390/cancers13215570