Rehabilitation after Allogeneic Haematopoietic Stem Cell Transplantation: A Special Challenge
Abstract
:Simple Summary
Abstract
1. Introduction
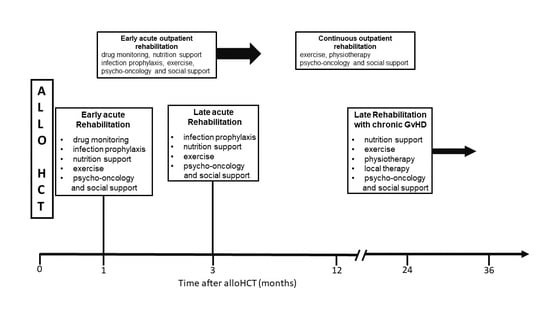
2. AlloHCT
3. Acute Rehabilitation as an Inpatient
3.1. Malnutrition
3.2. Muscle Loss
- Endurance;
- Strength;
- Balance.
3.3. Risk of Infections
- Early introduction of antibiotics reduces the risk of GvHD [36].
3.4. Psycho-Oncological Aspects
3.5. Psychosocial Aspects
3.6. Graft-versus-Host Disease
3.6.1. Acute GvHD (aGvHD)
3.6.2. Chronic GvHD (cGvHD)
3.7. Laboratory Diagnostics
4. Acute Rehabilitation as an Outpatient
- A compliant patient;
- The patient is mobile and can care for her/himself, or;
- Is well integrated and cared for by the family;
- Lives close to an alloHCT-experienced haematologist or;
- To the primary transplant centre’s outpatient department.
5. Acute Rehabilitation as an Inpatient Later in the Time Course
6. Rehabilitation with Chronic GvHD
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Maehr, B.; Keilani, M.; Wiltschke, C.; Hassler, M.; Licht, T.; Marosi, C.; Huetterer, E.; Cenik, F.; Crevenna, R. Cancer Rehabilitation in Austria—Aspects of Physical Medicine and Rehabilitation. Wien. Med. Wochenschr. 2016, 166, 39–43. [Google Scholar] [CrossRef]
- Silver, J.K.; Stout, N.L.; Fu, J.B.; Pratt-Chapman, M.; Haylock, P.J.; Sharma, R. The State of Cancer Rehabilitation in the United States. J. Cancer Rehabil. 2018, 1, 1–8. [Google Scholar]
- Hellbom, M.; Bergelt, C.; Bergenmar, M.; Gijsen, B.; Loge, J.H.; Rautalathi, M.; Smaradottir, A.; Johansen, C. Cancer Rehabilitation: A Nordic and European Perspective. Acta Oncol. 2011, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.; Bethge, W.A.; Schmoor, C.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Standard Graft-versus-Host Disease Prophylaxis with or without Anti-T-Cell Globulin in Haematopoietic Cell Transplantation from Matched Unrelated Donors: A Randomised, Open-Label, Multicentre Phase 3 Trial. Lancet Oncol. 2009, 10, 855–864. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Greinix, H.; Halter, J.P.; Lawitschka, A.; Bertz, H.; Wolff, D. Long-Term Follow-up After Allogeneic Stem Cell Transplantation. Dtsch. Aerzteblatt Online 2015, 112, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Snowden, J.A.; McGrath, E.; Duarte, R.F.; Saccardi, R.; Orchard, K.; Worel, N.; Kuball, J.; Chabannon, C.; Mohty, M. JACIE Accreditation for Blood and Marrow Transplantation: Past, Present and Future Directions of an International Model for Healthcare Quality Improvement. Bone Marrow Transpl. 2017, 52, 1367–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Lans, M.C.M.; Witkamp, F.E.; Oldenmenger, W.H.; Broers, A.E.C. Five Phases of Recovery and Rehabilitation After Allogeneic Stem Cell Transplantation: A Qualitative Study. Cancer Nurs. 2019, 42, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.Ø.; Friis, L.S.; Hoybye, M.T.; Marcher, C.W.; Hansen, D.G. Rehabilitation during Intensive Treatment of Acute Leukaemia Including Allogenic Stem Cell Transplantation: A Qualitative Study of Patient Experiences. BMJ Open 2019, 9, e029470. [Google Scholar] [CrossRef] [Green Version]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A Systematic Review of Rehabilitation and Exercise Recommendations in Oncology Guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Bertz, H.; Potthoff, K.; Finke, J. Allogeneic Stem-Cell Transplantation From Related and Unrelated Donors in Older Patients With Myeloid Leukaemia. J. Clin. Oncol. JCO 2003, 21, 1480–1484. [Google Scholar] [CrossRef]
- Marks, R.; Potthoff, K.; Hahn, J.; Ihorst, G.; Bertz, H.; Spyridonidis, A.; Holler, E.; Finke, J.M. Reduced-Toxicity Conditioning with Fludarabine, BCNU, and Melphalan in Allogeneic Hematopoietic Cell Transplantation: Particular Activity against Advanced Hematologic Malignancies. Blood 2008, 112, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Elko, T.A.; Devlin, S.M.; Shahrokni, A.; Jakubowski, A.A.; Dahi, P.B.; Perales, M.-A.; Tamari, R.; Shaffer, B.C.; Sauter, C.S.; et al. Impact of Geriatric Vulnerabilities on Allogeneic Hematopoietic Cell Transplantation Outcomes in Older Patients with Hematologic Malignancies. Bone Marrow Transpl. 2020, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lindman, A.; Handberg, C.; Olesen, G.; Duijts, S. Health-related Quality of Life and Physical Functioning in Patients Participating in a Rehabilitation Programme, Undergoing Non-myeloablative Allogeneic Haematopoietic Stem Cell Transplantation: Outcomes from a Single Arm Longitudinal Study. Eur. J. Cancer Care 2021, 30, e13478. [Google Scholar] [CrossRef] [PubMed]
- Rick, O.; Kalusche, E.-M.; Dauelsberg, T.; König, V.; Korsukéwitz, C.; Seifart, U. Reintegrating Cancer Patients Into the Workplace. Dtsch. Aerzteblatt Int. 2012, 109, 702. [Google Scholar] [CrossRef] [PubMed]
- Fauser, D.; Wienert, J.; Zomorodbakhsch, B.; Schmielau, J.; Biester, I.; Krüger, H.-U.; Presl, A.; Bethge, M. Work-Related Medical Rehabilitation in Cancer. Dtsch. Aerzteblatt Online 2019, 116, 592–599. [Google Scholar] [CrossRef]
- Lindman, A.; Petersen, A.K.; Olesen, G.; Handberg, C. Patients’ Experiences and Perspectives of Challenges and Needs Related to Non-myeloablative Stem Cell Transplantation: Involving Patients in Developing a Targeted Rehabilitation Programme. J. Clin. Nurs. 2019, 28, 1260–1272. [Google Scholar] [CrossRef]
- Herzberg, P.Y.; Lee, S.J.; Heussner, P.; Mumm, F.H.A.; Hilgendorf, I.; von Harsdorf, S.; Hemmati, P.; Rieger, K.; Greinix, H.T.; Freund, M.; et al. Personality Influences Quality-of-Life Assessments in Adult Patients after Allogeneic Hematopoietic SCT: Results from a Joint Evaluation of the Prospective German Multicenter Validation Trial and the Fred Hutchinson Cancer Research Center. Bone Marrow Transpl. 2013, 48, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Ayuk, F.; Beelen, D.W.; Bornhäuser, M.; Stelljes, M.; Zabelina, T.; Finke, J.; Kobbe, G.; Wolff, D.; Wagner, E.-M.; Christopeit, M.; et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukaemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Barğı, G.; Boşnak Güçlü, M.; Türköz Sucak, A.G. Differences in Pulmonary and Extra-Pulmonary Characteristics in Severely versus Non-Severely Fatigued Recipients of Allogeneic Hematopoietic Stem Cell Transplantation: A Cross-Sectional, Comparative Study. Hematology 2019, 24, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Syrjala, K.L.; Artherholt, S.B.; Kurland, B.F.; Langer, S.L.; Roth-Roemer, S.; Elrod, J.B.; Dikmen, S. Prospective Neurocognitive Function Over 5 Years After Allogeneic Hematopoietic Cell Transplantation for Cancer Survivors Compared With Matched Controls at 5 Years. J. Clin. Oncol. JCO 2011, 29, 2397–2404. [Google Scholar] [CrossRef]
- Mayo, S.; Messner, H.A.; Rourke, S.B.; Howell, D.; Victor, J.C.; Kuruvilla, J.; Lipton, J.H.; Gupta, V.; Kim, D.D.; Piescic, C.; et al. Relationship between Neurocognitive Functioning and Medication Management Ability over the First 6 Months Following Allogeneic Stem Cell Transplantation. Bone Marrow Transpl. 2016, 51, 841–847. [Google Scholar] [CrossRef]
- Urbain, P.; Birlinger, J.; Lambert, C.; Finke, J.; Bertz, H.; Biesalski, H.-K. Longitudinal Follow-up of Nutritional Status and Its Influencing Factors in Adults Undergoing Allogeneic Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2013, 48, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbain, P.; Birlinger, J.; Ihorst, G.; Biesalski, H.-K.; Finke, J.; Bertz, H. Body Mass Index and Bioelectrical Impedance Phase Angle as Potentially Modifiable Nutritional Markers Are Independent Risk Factors for Outcome in Allogeneic Hematopoietic Cell Transplantation. Ann. Hematol. 2013, 92, 111–119. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Toenges, R.; Greinix, H.; Lawitschka, A.; Halter, J.; Baumgartner, A.; Simon, A.; Arends, J.; Jäger, P.; Middeke, M.; Hilgendorf, I.; et al. Current Practice in Nutrition after Allogeneic Hematopoietic Stem Cell Transplantation–Results from a Survey among Hematopoietic Stem Cell Transplant Centers. Clin. Nutr. 2021, 40, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Limbach, M.; (Freiburg University, Freiburg, Germany). Personal communication, 2016.
- Dimeo, F.; Bertz, H.; Finke, J.; Fetscher, S.; Mertelsmann, R.; Keul, J. An Aerobic Exercise Program for Patients with Haematological Malignancies after Bone Marrow Transplantation. Bone Marrow Transpl. 1996, 18, 1157–1160. [Google Scholar]
- Pahl, A.; Wehrle, A.; Kneis, S.; Gollhofer, A.; Bertz, H. Feasibility of Whole Body Vibration during Intensive Chemotherapy in Patients with Hematological Malignancies–a Randomized Controlled Pilot Study. BMC Cancer 2018, 18, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiskemann, J.; Dreger, P.; Schwerdtfeger, R.; Bondong, A.; Huber, G.; Kleindienst, N.; Ulrich, C.M.; Bohus, M. Effects of a Partly Self-Administered Exercise Program before, during, and after Allogeneic Stem Cell Transplantation. Blood 2011, 117, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Pahl, A.; Wehrle, A.; Kneis, S.; Gollhofer, A.; Bertz, H. Whole Body Vibration Training during Allogeneic Hematopoietic Cell Transplantation—the Effects on Patients’ Physical Capacity. Ann. Hematol. 2020, 99, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Bewarder, M.; Klostermann, A.; Ahlgrimm, M.; Bittenbring, J.T.; Pfreundschuh, M.; Wagenpfeil, S.; Kaddu-Mulindwa, D. Safety and Feasibility of Electrical Muscle Stimulation in Patients Undergoing Autologous and Allogeneic Stem Cell Transplantation or Intensive Chemotherapy. Support. Care Cancer 2019, 27, 1013–1020. [Google Scholar] [CrossRef]
- Morishita, S.; Kaida, K.; Aoki, O.; Yamauchi, S.; Wakasugi, T.; Ikegame, K.; Ogawa, H.; Domen, K. Balance Function in Patients Who Had Undergone Allogeneic Hematopoietic Stem Cell Transplantation. Gait Posture 2015, 42, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Kneis, S.; Straub, E.; Walz, I.D.; von Olshausen, P.; Wehrle, A.; Gollhofer, A.; Bertz, H. Gait Analysis of Patients After Allogeneic Hematopoietic Cell Transplantation Reveals Impairments of Functional Performance. Integr. Cancer Ther. 2020, 19, 153473542091578. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, A.; Kneis, S.; Dickhuth, H.-H.; Gollhofer, A.; Bertz, H. Endurance and Resistance Training in Patients with Acute Leukaemia Undergoing Induction Chemotherapy—A Randomized Pilot Study. Support. Care Cancer 2019, 27, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Schmidt-Hieber, M.; Bertz, H.; Heinz, W.J.; Kiehl, M.; Krüger, W.; Mousset, S.; Neuburger, S.; Neumann, S.; Penack, O.; et al. Infectious Diseases in Allogeneic Haematopoietic Stem Cell Transplantation: Prevention and Prophylaxis Strategy Guidelines 2016. Ann. Hematol. 2016, 95, 1435–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuji, S.; Kapp, M.; Einsele, H. Possible Implication of Bacterial Infection in Acute Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Oncol. 2014, 4, 89. [Google Scholar] [CrossRef]
- Luft, D.; Schmoor, C.; Wilson, C.; Widmer, A.F.; Bertz, H.; Frei, R.; Heim, D.; Dettenkofer, M. Central Venous Catheter-Associated Bloodstream Infection and Colonisation of Insertion Site and Catheter Tip. What Are the Rates and Risk Factors in Haematology Patients? Ann. Hematol. 2010, 89, 1265–1275. [Google Scholar] [CrossRef] [Green Version]
- Fritzsche, K.; Struss, Y.; Hammel, A.; Bertz, H.; Stein, B. Relationship between Psychosocial Distress, Treatment Need and Use of Psychotherapeutic Interventions within a Psychosomatic Liaison Service in Hematological Oncology. Oncol. Res. Treat. 2004, 27, 457–461. [Google Scholar] [CrossRef]
- Morishita, S.; Kaida, K.; Yamauchi, S.; Wakasugi, T.; Ikegame, K.; Kodama, N.; Ogawa, H.; Domen, K. Early-Phase Differences in Health-Related Quality of Life, Psychological Status, and Physical Function between Human Leucocyte Antigen-Haploidentical and Other Allogeneic Haematopoietic Stem Cell Transplantation Recipients. Eur. J. Oncol. Nurs. 2015, 19, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Kaida, K.; Yamauchi, S.; Wakasugi, T.; Yoshihara, S.; Taniguchi, K.; Ishii, S.; Ikegame, K.; Kodama, N.; Ogawa, H.; et al. Gender Differences in Health-Related Quality of Life, Physical Function and Psychological Status among Patients in the Early Phase Following Allogeneic Haematopoietic Stem Cell Transplantation: Gender Differences of QOL in Allo-HSCT Patients. Psycho-Oncology 2013, 22, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Schettler, P.; Bresee, C. A Preliminary Study of the Effects of a Single Session of Swedish Massage on Hypothalamic–Pituitary–Adrenal and Immune Function in Normal Individuals. J. Altern. Complementary Med. 2010, 16, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Pulewka, K.; Wolff, D.; Herzberg, P.Y.; Greinix, H.; Heussner, P.; Mumm, F.H.A.; von Harsdorf, S.; Rieger, K.; Hemmati, P.; Hochhaus, A.; et al. Physical and Psychosocial Aspects of Adolescent and Young Adults after Allogeneic Hematopoietic Stem-Cell Transplantation: Results from a Prospective Multicenter Trial. J. Cancer Res. Clin. Oncol. 2017, 143, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R. Advances in Understanding the Pathogenesis of Graft-versus-host Disease. Br. J. Haematol. 2019, 187, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Ayuk, F.; Elmaagacli, A.; Bertz, H.; Lawitschka, A.; Schleuning, M.; Meyer, R.-G.; Gerbitz, A.; Hilgendorf, I.; Hildebrandt, G.C.; et al. Current Practice in Diagnosis and Treatment of Acute Graft-versus-Host Disease: Results from a Survey among German-Austrian-Swiss Hematopoietic Stem Cell Transplant Centers. Biol. Blood Marrow Transplant. 2013, 19, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, D.; Bertz, H.; Greinix, H.; Lawitschka, A.; Halter, J.; Holler, E. The Treatment of Chronic Graft-Versus-Host Disease. Dtsch. Aerzteblatt Int. 2011, 108, 732. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Pergolotti, M.; Covington, K.R.; Lightner, A.N.; Bertram, J.; Thess, M.; Sharp, J.; Spraker, M.; Williams, G.R.; Manning, P. Association of Outpatient Cancer Rehabilitation With Patient-Reported Outcomes and Performance-Based Measures of Function. Rehabil. Oncol. 2021, 39, 137–142. [Google Scholar] [CrossRef]
- Leppla, L.; Schmid, A.; Valenta, S.; Mielke, J.; Beckmann, S.; Ribaut, J.; Teynor, A.; Dobbels, F.; Duerinckx, N.; Zeiser, R.; et al. Development of an Integrated Model of Care for Allogeneic Stem Cell Transplantation Facilitated by EHealth—The SMILe Study. Support. Care Cancer 2021, 29, 8045–8057. [Google Scholar] [CrossRef] [PubMed]
- Leppla, L.; Mielke, J.; Kunze, M.; Mauthner, O.; Teynor, A.; Valenta, S.; Vanhoof, J.; Dobbels, F.; Berben, L.; Zeiser, R.; et al. Clinicians and Patients Perspectives on Follow-up Care and EHealth Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Mixed-Methods Contextual Analysis as Part of the SMILe Study. Eur. J. Oncol. Nurs. 2020, 45, 101723. [Google Scholar] [CrossRef]
- Fu, J.B.; Raj, V.S.; Guo, Y. A Guide to Inpatient Cancer Rehabilitation: Focusing on Patient Selection and Evidence-Based Outcomes. PM&R 2017, 9, S324–S334. [Google Scholar] [CrossRef]
- Dietrich-Ntoukas, T.; Cursiefen, C.; Westekemper, H.; Eberwein, P.; Reinhard, T.; Bertz, H.; Nepp, J.; Lawitschka, A.; Heiligenhaus, A.; Seitz, B.; et al. Diagnosis and Treatment of Ocular Chronic Graft-Versus-Host Disease: Report From the German–Austrian–Swiss Consensus Conference on Clinical Practice in Chronic GVHD. Cornea 2012, 31, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Grauer, O.; Wolff, D.; Bertz, H.; Greinix, H.; Kuhl, J.-S.; Lawitschka, A.; Lee, S.J.; Pavletic, S.Z.; Holler, E.; Kleiter, I. Neurological Manifestations of Chronic Graft-versus-Host Disease after Allogeneic Haematopoietic Stem Cell Transplantation: Report from the Consensus Conference on Clinical Practice in Chronic Graft-versus-Host Disease. Brain 2010, 133, 2852–2865. [Google Scholar] [CrossRef] [Green Version]
- Marks, C.; Stadler, M.; Häusermann, P.; Wolff, D.; Buchholz, S.; Stary, G.; Lee, S.; Lawitschka, A.; Bertz, H. German-Austrian-Swiss Consensus Conference on Clinical Practice in Chronic Graft-versus-Host Disease (GVHD): Guidance for Supportive Therapy of Chronic Cutaneous and Musculoskeletal GVHD: Consensus Conference on Clinical Practice in GVHD. Br. J. Dermatol. 2011, 165, 18–29. [Google Scholar] [CrossRef]
- Frey Tirri, B.; Häusermann, P.; Bertz, H.; Greinix, H.; Lawitschka, A.; Schwarze, C.-P.; Wolff, D.; Halter, J.P.; Dörfler, D.; Moffat, R. Clinical Guidelines for Gynecologic Care after Hematopoietic SCT. Report from the International Consensus Project on Clinical Practice in Chronic GVHD. Bone Marrow Transplant. 2015, 50, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.; Smith, S.; Kalpakjian, C. Functional Outcomes of Acute Inpatient Rehabilitation in Patients With Chronic Graft-Versus-Host Disease. PM&R 2018, 10, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.R.; Haig, A.J.; Couriel, D.R. Musculoskeletal, Neurologic, and Cardiopulmonary Aspects of Physical Rehabilitation in Patients with Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molés-Poveda, P.; Comis, L.E.; Joe, G.O.; Mitchell, S.A.; Pichard, D.C.; Rosenstein, R.K.; Solomon, B.; Pavletic, S.Z.; Cowen, E.W. Rehabilitation Interventions in the Multidisciplinary Management of Patients With Sclerotic Graft-Versus-Host Disease of the Skin and Fascia. Arch. Phys. Med. Rehabil. 2021, 102, 776–788. [Google Scholar] [CrossRef]
- Tran, J.; Norder, E.E.; Diaz, P.T.; Phillips, G.S.; Elder, P.; Devine, S.M.; Wood, K.L. Pulmonary Rehabilitation for Bronchiolitis Obliterans Syndrome after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1250–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Jawahri, A.; Fishman, S.R.; Vanderklish, J.; Dizon, D.S.; Pensak, N.; Traeger, L.; Greer, J.A.; Park, E.R.; Markovitz, N.; Waldman, L.; et al. Pilot Study of a Multimodal Intervention to Enhance Sexual Function in Survivors of Hematopoietic Stem Cell Transplantation: Intervention for Sexual Dysfunction in HCT. Cancer 2018, 124, 2438–2446. [Google Scholar] [CrossRef]
- Mohananey, D.; Sarau, A.; Kumar, R.; Lewandowski, D.; Abreu-Sosa, S.M.; Nathan, S.; Okwuosa, T.M. Role of Physical Activity and Cardiac Rehabilitation in Patients Undergoing Hematopoietic Stem Cell Transplantation. JACC CardioOncology 2021, 3, 17–34. [Google Scholar] [CrossRef]
- Wong, F.L.; Francisco, L.; Togawa, K.; Bosworth, A.; Gonzales, M.; Hanby, C.; Sabado, M.; Grant, M.; Forman, S.J.; Bhatia, S. Long-Term Recovery after Hematopoietic Cell Transplantation: Predictors of Quality-of-Life Concerns. Blood 2010, 115, 2508–2519. [Google Scholar] [CrossRef]
- Frödin, U.; Lotfi, K.; Fomichov, V.; Juliusson, G.; Börjeson, S. Frequent and Long-Term Follow-up of Health-Related Quality of Life Following Allogeneic Haematopoietic Stem Cell Transplantation. Eur. J. Cancer Care 2015, 24, 898–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Disease |
|---|
|
|
| Main Side Effects at Admission to Rehabilitation |
|---|
|
| Food to Avoid until Minimum Day 180 or Full Immune Reconstitution, Whichever Comes First |
|---|
| raw fish (sushi) raw milk and raw milk products raw meat (steak tartar, ground pork) carpaccio shellfish/crustaceans raw grain fresh or raw seeds or grains raw eggs mouldy food, blue cheeses soft ice grapefruit, pomelos, and pomegranates (they interfere with immunosuppressive drugs) |
| Important Forms of Exercise |
|---|
|
| Pathogen | Drug Prophylaxis | Laboratory Diagnostic |
|---|---|---|
| CMV | Letermovir | CMV PCR weekly |
| EBV | No | EBV PCR weekly |
| HSV | Aciclovir/valaciclovir | HSV PCR if infect is suspected |
| VZV | Aciclovir/valaciclovir | VZV PCR if infect is suspected |
| Toxoplasmosis | Trimethoprim-sulfamethoxazole | no |
| Pneumocystis jirovecii | Trimethoprim-sulfamethoxazole. | no |
| Tuberculosis | INH + Vitamin B6 | no |
| Hepatitis B | Lamivudine or entecavir | HBV PCR if reactivation is suspected |
| Virus | Kind of Vaccination | Recommendation | Time Points after HCT | Antibody Titer before Vaccination |
|---|---|---|---|---|
| Tetanus | inactivated | recommended | month (6–)12 | not recommended |
| Diphtheria | inactivated | recommended | month (6–)12 | not recommended |
| Poliomyelitis | inactivated | recommended | month (6–)12 | not recommended |
| Pneumococcus | inactivated | recommended | month (6–)12 | not recommended |
| Pertussis | inactivated | recommended | month (6–)12 | not recommended |
| Influenza | inactivated | recommended | >month 3, seasonal | not recommended |
| Haemophilus influenzae | inactivated | recommended | month (6–)12 | not recommended |
| Meningococci | inactivated | recommended | month (6–)12 | not recommended |
| SARS-CoV-19 | inactivated | recommended | >month 3 | unclear |
| Hepatitis A/B | inactivated | recommended | month (6–)12 | not recommended |
| HPV | inactivated | recommended | month (6–)12 | not recommended |
| Tick-borne | inactivated | recommended | month (6–)12 | not recommended |
| Varicella | inactivated | recommended | month (6–)12 | not recommended |
| Mumps | live vaccine | case-by-case decision | 2 years; w/o IS | before vaccination |
| Measles | live vaccine | case-by-case decision | 2 years; w/o IS | before vaccination |
| Rubella | live vaccine | case-by-case decision | 2 years; w/o IS | before vaccination |
| Varicella | live vaccine | case-by-case decision | 2 years; w/o IS | not recommended |
| Lab-Controls Minimum Once a Week |
|---|
| white blood cell count, neutrophils, haemoglobin, platelets |
| potassium, sodium chloride, magnesium |
| ALT, gamma-GT |
| INR |
| C reactive protein |
| protein, serum albumin |
| immunosuppression blood levels |
| Special Exercises |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertz, H. Rehabilitation after Allogeneic Haematopoietic Stem Cell Transplantation: A Special Challenge. Cancers 2021, 13, 6187. https://doi.org/10.3390/cancers13246187
Bertz H. Rehabilitation after Allogeneic Haematopoietic Stem Cell Transplantation: A Special Challenge. Cancers. 2021; 13(24):6187. https://doi.org/10.3390/cancers13246187
Chicago/Turabian StyleBertz, Hartmut. 2021. "Rehabilitation after Allogeneic Haematopoietic Stem Cell Transplantation: A Special Challenge" Cancers 13, no. 24: 6187. https://doi.org/10.3390/cancers13246187
APA StyleBertz, H. (2021). Rehabilitation after Allogeneic Haematopoietic Stem Cell Transplantation: A Special Challenge. Cancers, 13(24), 6187. https://doi.org/10.3390/cancers13246187