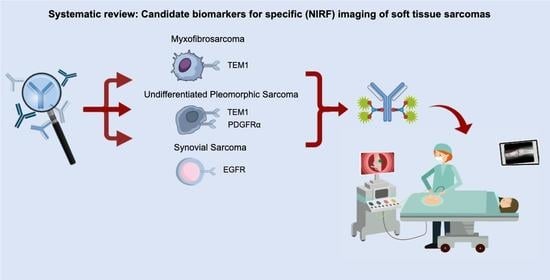
Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Biomarker Selection Scoring System
- Sample size. The number of samples indicate how much evidence is acquired.
- Percentage of biomarker-positive STS samples. This is calculated based on the amount of STS samples that positively showed presence of the biomarker in each included article, independent of the percentage of positive tumor cells within each sample. Immunohistochemistry was used to assess the percentage of positive STS in tissue samples.
- Pattern of expression. Ideally, the target is expressed diffusely by all tumor cells (particularly at the tumor border) to guide surgical resection. The pattern of expression is defined as diffuse when expression is randomly spread throughout the tumor sample and focal when expression is located in a specific region of the tumor sample. When different samples show variable expression patterns (diffuse and focal), the expression pattern for the whole cohort is defined as heterogeneous. No distinction was made based on exact location of expression within tumor samples. While this review included studies evaluating tissue samples and tissue microarrays, data regarding the pattern of expression was extracted from studies including tissue samples.
- Internalization. This is important because internalization after binding of the tracer creates a long-lasting signal for tumor-specific imaging.
- Previously imaged. If there is prove that imaging is possible, it has more potential to be translated to the clinics. The distinction between imaging with or without NIRF is important for its applicability in NIRF imaging. This criterium was tumor type independent.
3. Results
3.1. Study Selection
3.2. Candidate Biomarkers
3.2.1. TEM1
3.2.2. VEGFR
3.2.3. EGFR
3.2.4. IGF-1R
3.2.5. PDGFR
3.2.6. CD40
3.3. Potential NIRF Imaging Tracers Safety Profile
4. Discussion
4.1. Research Aim
4.2. Comparing the Selected Biomarkers
4.3. MFS, USTS and SS
4.4. Comparison of Potential NIRF Imaging Tracers
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy
Appendix B. Search Previously Imaged and Search Internalization
Appendix C. STS Subtypes Examined for Each of the Top 7 Biomarkers
| Biomarker | STS Subtypes |
|---|---|
| TEM1 | Angiosarcoma, desmoplastic small round cell, epithelioid haemangioendothelioma, epithelioid sarcoma, fibrosarcoma, inflammatory myofibroblastic sarcoma, kaposi sarcoma, LMS, liposarcoma, MPNST, malignant solitary fibrous tumor, myxofibrosarcoma, RMS, spindle cell sarcoma NOS, synovial sarcoma, USTS, and uterine sarcoma |
| VEGFR-1 | Alveolar STS, angiosarcoma, endometrial stromal sarcoma, Kaposi sarcoma, LMS, liposarcoma, MPNST, malignant solitary fibrous tumor, myxofibrosarcoma, myxoid liposarcoma, pulmonary artery sarcoma, RMS, sarcoma NOS, synovial sarcoma, and USTS |
| VEGFR-2 | Alveolar STS, angiosarcoma, endometrial stromal sarcoma, epithelioid hemangioendotheliomas, fibrosarcoma, LMS, liposarcoma, MPNST, malignant solitary fibrous tumor, myxofibrosarcoma, pulmonary artery sarcoma, RMS, sarcoma NOS, synovial sarcoma, and USTS |
| EGFR | Acral myxoinflammatory fibroblastic sarcoma, alveolar soft part sarcoma, atypical fibroxanthoma, desmoplastic tumor, endometrial stromal sarcoma, epithelioid sarcoma, fibromatosis, fibromyxoid sarcoma, fibrosarcoma, follicular dendritic cell sarcoma, intimal sarcoma, liposarcoma, LMS, MPNST, myofibroblastic sarcoma, myoxyoinflammatory fibroblastic sarcoma, myxofibrosarcoma, myxoid lipsarcoma, myxoid sarcoma, pleomorphic dermal sarcoma, RMS, sarcoma NOS, synovial sarcoma, endifferentiated endometrial sarcoma, USTS, and undifferentiated stromal sarcoma |
| IGF-1R | Alveolar STS, angiosarcoma, desmoplastic tumor, fibrosarcoma, LMS, liposarcoma, MPNST, mesenchyoma, myxofibrosarcoma, RMS, sarcoma NOS, spindle cell sarcoma, synovial sarcoma, and USTS |
| PDGFRα | Alveolar soft part sarcoma, Angiosarcoma, dermatofibrosarcoma protuberans, endometrial stromal sarcoma, fibromyxoid sarcoma, fibrosarcoma, liposarcoma, LMS, MPNST, myofibroblastic sarcoma, myoxyoinflammatory fibroblastic sarcoma, myxofibrosarcoma, myxoid liposarcoma, pulmonary artery sarcoma, RMS, sarcoma NOS, solitary fibrous tumor, synovial sarcoma, undifferentiated endometrial sarcoma, undifferentiated uterine sarcoma, undifferentiated stromal sarcoma, and USTS |
| CD40 | Kaposi sarcoma, liposarcoma, LMS, MPNST, RMS, and USTS |
Appendix D. Toxicity of Clinically Available Monoclonal Antibodies in Patients with STS
| Clinical Trial | Phase | Tumor Type | Evaluable for Toxicity | Median a,* Age (Years) | Treatment | Most Common Adverse Events | Percentage Patients with ≥3 Adverse Events (vs. Placebo) | Most Common Grade ≥ 3 Adverse Events |
|---|---|---|---|---|---|---|---|---|
| TEM1 (Ontuxizumab) | ||||||||
| Jones et al., 2019 | 2 | STS | 209 | 55 | Ontuxizumab 8mg/kg + G/D vs. placebo + G/D b | Fatigue (74% vs. 66%), anemia (61% vs. 60%), nausea (56% vs. 52%), diarrhea (44% vs. 36%), and peripheral edema (42% vs. 45%) | Not reported | Pyrexia (4% vs. 0%) and anemia (1% vs. 3%) c |
| IGF-1R (Teprotomumab, Figitumumab and Cixutumumab) | ||||||||
| Pappo et al., 2014 | 2 | STS + osteosarcoma | 163 | 31 | Teprotumumab 9 mg/kg; 1 dose per week | Fatigue (20.2%), nausea (14.1%), hyperglycemia (9.2%), and muscle spasms (8.6%) | 10.4% | Hyperglycemia (2.5%), dehydration (1.8%), fatigue (1.8%), and hyponatremia (1.2%) |
| Olmos et al., 2010 | 1 | STS + Ewing sarcoma + myxoid chondrosarcoma | 29 | 30 | Figitumumab 20 mg/kg; 1 dose per 3–4 weeks | Hyperglycemia (17%), skin reactions (rash, urticaria, infection, eczema) (13.8%), increased GGT (10.3%), headache (10.3%), and fatigue (10.3%) | 17.2% | Vomiting (3.4%), back pain (3.4%), DVT (3.4%), increased uric acid concentration (3.4%), and increased AST, ALT or GGT (3.4%) |
| Wagner et al., 2015 | 2 | STS + Ewing sarcoma + osteosarcoma | 44 | 14–181 | Cixutumumab 6 mg/kg and Temsirolimus 8 mg/m2; 1 dose per week | Mucositis, electrolyte disturbances and myelosuppression | Not reported | Neutropenia (13.6%), thrombocytopenia (11.4%), hypokalemia (11.4%), oral mucositis (9.1%), and hypophosphatemia (9.1%) |
| Schöffski et al., 2013 | 2 | STS + Ewing family of tumors | 113 | 27.5–33.1 | Cixutumumab 10 mg/kg; 1 dose per 2 weeks | Nausea (26.1%), fatigue (23.4%), diarrhea (22.5%), hyperglycemia (19.8%), and anorexia (17.1%) | Not reported | Hyperglycemia (5.4%), pain (5.4%), thrombocytopenia (4.5%), asthenia (4.5%), and anemia (3.6%) |
| Schwartz et al., 2013 | 2 | STS + sarcoma of bone | 174 | Mean: 48.1 | Cixutumumab 6 mg/kg and Temsirolimus 25 mg; 1 dose per week | Oral mucositis (71.3%), hypercalcemia (68.4%), fatigue (65.5%), thrombocytopenia (63.8%), and anemia (62.6%) | Not reported | Anemia (9%), hyperglycemia (10%), hypophosphatemia (9%), lymphopenia (14%), oral mucositis (11%), and thrombocytopenia (11%) |
| PDGFR (Olaratumab) | ||||||||
| Tap et al., 2020 | 3 | STS | 506 | Mean: 56.9 | Olaratumab 15 mg/kg + Doxorubicin 75 mg/m2 vs. Placebo + Doxorubicin 75 mg/m2 d | Nausea (59.5% vs. 66.7%), neutropenia (55.3% vs. 57.8%), fatigue (54.1% vs. 59%), alopecia (43.6% vs. 49.8%), and anemia (42.8% vs. 45.4%) | Not reported | Neutropenia (46.3% vs. 49%), leukopenia (23.3% vs. 23.7%), febrile neutropenia (17.5% vs. 16.5%), anemia (13.6% vs. 12.4%), and thrombocytopenia (9.3% vs. 8.4%) |
| Yonemori et al., 2018 | 1 | STS | 19 | 41.5–52 a | Olaratumab 15 mg/kg + Doxorubicin 25-75 mg/m2 e | ALT increased (52.6%), neutrophil count decreased (52.6%), WBC count decreased (47.4%), anemia (36.8%), and GGT increased (31.6%) | 57.9% | Decreased neutrophil count (42.1%), decreased WBC count (42.1%), increased ALT (15.8%), anemia (10.5%), and febrile neutropenia (10.5%) |
| Tap et al., 2016 | 2 | STS | 133 | 58.5 | Olaratumab 15 mg/kg + Doxorubicin 75 mg/m2 vs. Doxorubicin f | Nausea (73.4% vs. 52.3%), fatigue (68.8% vs. 69.2%), neutropenia (57.8% vs. 35.4%), mucositis (53.1% vs. 35.4%), and alopecia (51.6% vs. 40%) | 67% vs. 55% | Neutropenia (53.2% vs. 32.3%), leukopenia (36% vs. 16.9%), febrile neutropenia (12.5% vs. 13.8%), anemia (12.5% vs. 9.2%), and fatigue (9.4% vs. 3.1%) |
References
- Vos, M.; Blaauwgeers, H.G.T.; Ho, V.K.Y.; van Houdt, W.J.; van der Hage, J.A.; Been, L.B.; Bonenkamp, J.J.; Bemelmans, M.H.A.; van Dalen, T.; Haas, R.L.; et al. Increased survival of non low-grade and deep-seated soft tissue sarcoma after surgical management in high-volume hospitals: A nationwide study from the Netherlands. Eur. J. Cancer 2019, 110, 98–106. [Google Scholar] [CrossRef] [PubMed]
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii102–iii112. [Google Scholar] [CrossRef]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W., Jr.; Donegan, W.L.; Natarajan, N.; Mettlin, C.; Beart, R.; Winchester, D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann. Surg. 1987, 205, 349–359. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours. Tissue and Bone Tumours: WHO Classification of Tumours, 5th ed.; IARC Publications: Lyon, France, 2020; Volume 3. [Google Scholar]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980. [Google Scholar] [CrossRef]
- Pasquali, S.; Colombo, C.; Pizzamiglio, S.; Verderio, P.; Callegaro, D.; Stacchiotti, S.; Martin Broto, J.; Lopez-Pousa, A.; Ferrari, S.; Poveda, A.; et al. High-risk soft tissue sarcomas treated with perioperative chemotherapy: Improving prognostic classification in a randomised clinical trial. Eur. J. Cancer 2018, 93, 28–36. [Google Scholar] [CrossRef]
- Smolle, M.A.; Sande, M.V.; Callegaro, D.; Wunder, J.; Hayes, A.; Leitner, L.; Bergovec, M.; Tunn, P.U.; van Praag, V.; Fiocco, M.; et al. Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers 2019, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Willeumier, J.; Fiocco, M.; Nout, R.; Dijkstra, S.; Aston, W.; Pollock, R.; Hartgrink, H.; Bovee, J.; van de Sande, M. High-grade soft tissue sarcomas of the extremities: Surgical margins influence only local recurrence not overall survival. Int. Orthop. 2015, 39, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Kandel, R.; Coakley, N.; Werier, J.; Engel, J.; Ghert, M.; Verma, S. Surgical margins and handling of soft-tissue sarcoma in extremities: A clinical practice guideline. Curr. Oncol. 2013, 20, e247–e254. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Kang, Y.; Choi, J.Y.; Moon, K.C.; Kim, H.S.; Han, I.; Yi, M.; Kang, H.S. MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur. Radiol. 2014, 24, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.A.; Beard, J.A.S.; Grimer, R.J. Should Soft Tissue Sarcomas be Treated at a Specialist Centre? Sarcoma 2004, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Huang, W.; Luo, P.; Cai, W.; Yang, L.; Sun, Z.; Zheng, B.; Yan, W.; Wang, C. Undifferentiated Pleomorphic Sarcoma: Long-Term Follow-Up from a Large Institution. Cancer Manag. Res. 2019, 11, 10001–10009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.J.; Antonescu, C.R.; Leung, D.H.; Blumberg, D.; Healey, J.H.; Woodruff, J.M.; Brennan, M.F. Synovial sarcoma: A multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J. Clin. Oncol. 2000, 18, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Look Hong, N.J.; Hornicek, F.J.; Raskin, K.A.; Yoon, S.S.; Szymonifka, J.; Yeap, B.; Chen, Y.L.; DeLaney, T.F.; Nielsen, G.P.; Mullen, J.T. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann. Surg. Oncol. 2013, 20, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Odei, B.; Rwigema, J.C.; Eilber, F.R.; Eilber, F.C.; Selch, M.; Singh, A.; Chmielowski, B.; Nelson, S.D.; Wang, P.C.; Steinberg, M.; et al. Predictors of Local Recurrence in Patients With Myxofibrosarcoma. Am. J. Clin. Oncol. 2018, 41, 827–831. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet. Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Keereweer, S.; Van Driel, P.B.; Snoeks, T.J.; Kerrebijn, J.D.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.; Lowik, C.W. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [Green Version]
- Barth, C.W.; Gibbs, S.L. Fluorescence Image-Guided Surgery—A Perspective on Contrast Agent Development. Proc. Spie. Int. Soc. Opt. Eng. 2020, 11222. [Google Scholar] [CrossRef]
- Karaman, S.; Leppanen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosma, S.E.; van Driel, P.B.; Hogendoorn, P.C.; Dijkstra, P.S.; Sier, C.F. Introducing fluorescence guided surgery into orthopedic oncology: A systematic review of candidate protein targets for Ewing sarcoma. J. Surg. Oncol. 2018, 118, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, S.E.; Zheleznyak, A.; Studer, M.; O’Shannessy, D.J.; Lapi, S.E.; Van Tine, B.A. Development of 89Zr-Ontuxizumab for in vivo TEM-1/endosialin PET applications. Oncotarget 2016, 7, 13082–13092. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Hu, J.; Wang, Y.; Peng, X.; Min, J.; Wang, J.; Matthaiou, E.; Cheng, Y.; Sun, K.; Tong, X.; et al. Tumour endothelial marker 1/endosialin-mediated targeting of human sarcoma. Eur. J. Cancer 2018, 90, 111–121. [Google Scholar] [CrossRef]
- De Gooyer, J.M.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.S.; Frielink, C.; Desar, I.M.E.; de Wilt, J.H.W.; Flucke, U.; Rijpkema, M. Immunohistochemical selection of biomarkers for tumor-targeted image-guided surgery of myxofibrosarcoma. Sci. Rep. 2020, 10, 2915. [Google Scholar] [CrossRef] [Green Version]
- Rouleau, C.; Curiel, M.; Weber, W.; Smale, R.; Kurtzberg, L.; Mascarello, J.; Berger, C.; Wallar, G.; Bagley, R.; Honma, N.; et al. Endosialin protein expression and therapeutic target potential in human solid tumors: Sarcoma versus carcinoma. Clin. Cancer Res. 2008, 14, 7223–7236. [Google Scholar] [CrossRef] [Green Version]
- Thway, K.; Robertson, D.; Jones, R.L.; Selfe, J.; Shipley, J.; Fisher, C.; Isacke, C.M. Endosialin expression in soft tissue sarcoma as a potential marker of undifferentiated mesenchymal cells. Br. J. Cancer 2016, 115, 473–479. [Google Scholar] [CrossRef]
- Zhang, J.; Razavian, M.; Tavakoli, S.; Nie, L.; Tellides, G.; Backer, J.M.; Backer, M.V.; Bender, J.R.; Sadeghi, M.M. Molecular imaging of vascular endothelial growth factor receptors in graft arteriosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- Saban, M.R.; Backer, J.M.; Backer, M.V.; Maier, J.; Fowler, B.; Davis, C.A.; Simpson, C.; Wu, X.R.; Birder, L.; Freeman, M.R.; et al. VEGF receptors and neuropilins are expressed in the urothelial and neuronal cells in normal mouse urinary bladder and are upregulated in inflammation. Am. J. Physiol. Ren. Physiol. 2008, 295, F60–F72. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.K.; Goransson, M.; Olofsson, A.; Andersson, C.; Aman, P. Nuclear expression of FLT1 and its ligand PGF in FUS-DDIT3 carrying myxoid liposarcomas suggests the existence of an intracrine signaling loop. BMC Cancer 2010, 10, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, S.; Kikkawa, F.; Kajiyama, H.; Shibata, K.; Kawai, M.; Mizuno, K.; Nagasaka, T.; Ino, K.; Nomura, S. Prognostic importance of vascular endothelial growth factor and its receptors in the uterine sarcoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2005, 15, 329–336. [Google Scholar] [CrossRef]
- Gaumann, A.; Strubel, G.; Bode-Lesniewska, B.; Schmidtmann, I.; Kriegsmann, J.; Kirkpatrick, C.J. The role of tumor vascularisation in benign and malignant cardiovascular neoplasms: A comparison of cardiac myxoma and sarcomas of the pulmonary artery. Oncol. Rep. 2008, 20, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itakura, E.; Yamamoto, H.; Oda, Y.; Tsuneyoshi, M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J. Surg. Oncol. 2008, 97, 74–81. [Google Scholar] [CrossRef]
- Kampmann, E.; Altendorf-Hofmann, A.; Gibis, S.; Lindner, L.H.; Issels, R.; Kirchner, T.; Knosel, T. VEGFR2 predicts decreased patients survival in soft tissue sarcomas. Pathol. Res. Pract. 2015, 211, 726–730. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, J.G.; Chien, Y.T.; Lin, S.S.; Hsu, F.T. Suppression of ERK/NF-κB Activation Is Associated With Amentoflavone-Inhibited Osteosarcoma Progression In Vivo. Anticancer Res. 2019, 39, 3669–3675. [Google Scholar] [CrossRef]
- Yonemori, K.; Tsuta, K.; Ando, M.; Hirakawa, A.; Hatanaka, Y.; Matsuno, Y.; Chuman, H.; Yamazaki, N.; Fujiwara, Y.; Hasegawa, T. Contrasting prognostic implications of platelet-derived growth factor receptor-beta and vascular endothelial growth factor receptor-2 in patients with angiosarcoma. Ann. Surg. Oncol. 2011, 18, 2841–2850. [Google Scholar] [CrossRef]
- Young, R.J.; Woll, P.J.; Staton, C.A.; Reed, M.W.; Brown, N.J. Vascular-targeted agents for the treatment of angiosarcoma. Cancer Chemother. Pharmacol. 2014, 73, 259–270. [Google Scholar] [CrossRef]
- Harding, J.; Burtness, B. Cetuximab: An epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today 2005, 41, 107–127. [Google Scholar] [CrossRef]
- Samkoe, K.S.; Sardar, H.S.; Bates, B.D.; Tselepidakis, N.N.; Gunn, J.R.; Hoffer-Hawlik, K.A.; Feldwisch, J.; Pogue, B.W.; Paulsen, K.D.; Henderson, E.R. Preclinical imaging of epidermal growth factor receptor with ABY-029 in soft-tissue sarcoma for fluorescence-guided surgery and tumor detection. J. Surg. Oncol. 2019, 119, 1077–1086. [Google Scholar] [CrossRef]
- Anderson, S.E.; Nonaka, D.; Chuai, S.; Olshen, A.B.; Chi, D.; Sabbatini, P.; Soslow, R.A. p53, epidermal growth factor, and platelet-derived growth factor in uterine leiomyosarcoma and leiomyomas. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2006, 16, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Armistead, P.M.; Salganick, J.; Roh, J.S.; Steinert, D.M.; Patel, S.; Munsell, M.; El-Naggar, A.K.; Benjamin, R.S.; Zhang, W.; Trent, J.C. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma: Correlation with overall survival in 105 patients. Cancer 2007, 110, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.H.; Park, J.Y.; Rhim, C.C.; Kim, J.H.; Park, Y.; Kim, K.R.; Nam, J.H. Investigation of New Therapeutic Targets in Undifferentiated Endometrial Sarcoma. Gynecol. Obstet. Investig. 2017, 82, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, G.; Schmeler, K.M.; Coleman, R.L.; Tu, X.; Liu, J.; Kavanagh, J.J. Recurrence patterns and prognosis of endometrial stromal sarcoma and the potential of tyrosine kinase-inhibiting therapy. Gynecol. Oncol. 2011, 121, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cossu-Rocca, P.; Contini, M.; Uras, M.G.; Muroni, M.R.; Pili, F.; Carru, C.; Bosincu, L.; Massarelli, G.; Nogales, F.F.; De Miglio, M.R. Tyrosine kinase receptor status in endometrial stromal sarcoma: An immunohistochemical and genetic-molecular analysis. Int. J. Gynecol. Pathol. 2012, 31, 570–579. [Google Scholar] [CrossRef]
- Cuppens, T.; Annibali, D.; Coosemans, A.; Trovik, J.; Ter Haar, N.; Colas, E.; Garcia-Jimenez, A.; Van de Vijver, K.; Kruitwagen, R.P.; Brinkhuis, M.; et al. Potential Targets’ Analysis Reveals Dual PI3K/mTOR Pathway Inhibition as a Promising Therapeutic Strategy for Uterine Leiomyosarcomas-an ENITEC Group Initiative. Clin. Cancer. Res. 2017, 23, 1274–1285. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.; Ghadimi, M.P.; Demicco, E.G.; Creighton, C.J.; Torres, K.; Colombo, C.; Peng, T.; Lusby, K.; Ingram, D.; Hornick, J.L.; et al. Localized and metastatic myxoid/round cell liposarcoma: Clinical and molecular observations. Cancer 2013, 119, 1868–1877. [Google Scholar] [CrossRef]
- Iwasaki, S.; Sudo, T.; Miwa, M.; Ukita, M.; Morimoto, A.; Tamada, M.; Ueno, S.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; et al. Endometrial stromal sarcoma: Clinicopathological and immunophenotypic study of 16 cases. Arch. Gynecol. Obs. 2013, 288, 385–391. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, K.R.; Nam, J.H. Immunohistochemical analysis for therapeutic targets and prognostic markers in low-grade endometrial stromal sarcoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 81–89. [Google Scholar] [CrossRef]
- Ruping, K.; Altendorf-Hofmann, A.; Chen, Y.; Kampmann, E.; Gibis, S.; Lindner, L.; Katenkamp, D.; Petersen, I.; Knosel, T. High IGF2 and FGFR3 are associated with tumour progression in undifferentiated pleomorphic sarcomas, but EGFR and FGFR3 mutations are a rare event. J. Cancer Res. Clin. Oncol 2014, 140, 1315–1322. [Google Scholar] [CrossRef]
- Barbashina, V.; Benevenia, J.; Aviv, H.; Tsai, J.; Patterson, F.; Aisner, S.; Cohen, S.; Fernandes, H.; Skurnick, J.; Hameed, M. Oncoproteins and proliferation markers in synovial sarcomas: A clinicopathologic study of 19 cases. J. Cancer Res. Clin. Oncol. 2002, 128, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.J.; O’Donnell, R.J.; Horvai, A.E. Epithelioid sarcoma expresses epidermal growth factor receptor but gene amplification and kinase domain mutations are rare. Mod. Pathol. 2010, 23, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.M.; Memoli, V.A.; Gonzalez, R.S. Cell cycle and apoptosis regulatory proteins, proliferative markers, cell signaling molecules, CD209, and decorin immunoreactivity in low-grade myxofibrosarcoma and myxoma. Virchows Arch. 2015, 467, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, B.; Floris, G.; Finalet-Ferreiro, J.; Fletcher, C.D.; Coindre, J.M.; Guillou, L.; Hogendoorn, P.C.; Wozniak, A.; Vanspauwen, V.; Schoffski, P.; et al. Coactivated platelet-derived growth factor receptor {alpha} and epidermal growth factor receptor are potential therapeutic targets in intimal sarcoma. Cancer Res. 2010, 70, 7304–7314. [Google Scholar] [CrossRef] [Green Version]
- Ganti, R.; Skapek, S.X.; Zhang, J.; Fuller, C.E.; Wu, J.; Billups, C.A.; Breitfeld, P.P.; Dalton, J.D.; Meyer, W.H.; Khoury, J.D. Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonal rhabdomyosarcoma. Mod. Pathol. 2006, 19, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Kubat, J.S.; Fulton, R.S.; Anthony, A.T.; Combs, M.; Powell, C.B.; Littell, R.D. Clinical outcomes and prognostic markers in uterine leiomyosarcoma: A population-based cohort. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 622–628. [Google Scholar] [CrossRef]
- Gusterson, B.; Cowley, G.; McIlhinney, J.; Ozanne, B.; Fisher, C.; Reeves, B. Evidence for increased epidermal growth factor receptors in human sarcomas. Int. J. Cancer 1985, 36, 689–693. [Google Scholar] [CrossRef]
- Helbig, D.; Ihle, M.A.; Putz, K.; Tantcheva-Poor, I.; Mauch, C.; Buttner, R.; Quaas, A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget 2016, 7, 21763–21774. [Google Scholar] [CrossRef]
- Kovarik, C.L.; Barrett, T.; Auerbach, A.; Cassarino, D.S. Acral myxoinflammatory fibroblastic sarcoma: Case series and immunohistochemical analysis. J. Cutan. Pathol. 2008, 35, 192–196. [Google Scholar] [CrossRef]
- Leibl, S.; Moinfar, F. Mammary NOS-type sarcoma with CD10 expression: A rare entity with features of myoepithelial differentiation. Am. J. Surg. Pathol. 2006, 30, 450–456. [Google Scholar] [CrossRef]
- Moinfar, F.; Gogg-Kamerer, M.; Sommersacher, A.; Regitnig, P.; Man, Y.G.; Zatloukal, K.; Denk, H.; Tavassoli, F.A. Endometrial stromal sarcomas frequently express epidermal growth factor receptor (EGFR, HER-1): Potential basis for a new therapeutic approach. Am. J. Surg. Pathol. 2005, 29, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Asmane, I.; Watkin, E.; Alberti, L.; Duc, A.; Marec-Berard, P.; Ray-Coquard, I.; Cassier, P.; Decouvelaere, A.V.; Ranchere, D.; Kurtz, J.E.; et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: A predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur. J. Cancer 2012, 48, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Espina, V.; Chiechi, A.; Magagnoli, G.; Novello, C.; Pazzaglia, L.; Quattrini, I.; Picci, P.; Liotta, L.A.; Benassi, M.S. Mapping protein signal pathway interaction in sarcoma bone metastasis: Linkage between rank, metalloproteinases turnover and growth factor signaling pathways. Clin. Exp. Metastasis 2014, 31, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.J.; Lahat, G.; Myers, S.E.; Smith, K.D.; Zou, C.; Wang, W.L.; Lopez-Terrada, D.; Lev, D. Validation of potential therapeutic targets in alveolar soft part sarcoma: An immunohistochemical study utilizing tissue microarray. Histopathology 2009, 55, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Wada, T.; Kawai, A.; Yamaguchi, U.; Makimoto, A.; Kokai, Y.; Yamashita, T.; Chuman, H.; Beppu, Y.; Tani, Y.; et al. Expression of epidermal growth factor receptor, ERBB2 and KIT in adult soft tissue sarcomas: A clinicopathologic study of 281 cases. Cancer 2005, 103, 1881–1890. [Google Scholar] [CrossRef]
- Sun, X.; Chang, K.C.; Abruzzo, L.V.; Lai, R.; Younes, A.; Jones, D. Epidermal growth factor receptor expression in follicular dendritic cells: A shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum. Pathol. 2003, 34, 835–840. [Google Scholar] [CrossRef]
- Tamborini, E.; Casieri, P.; Miselli, F.; Orsenigo, M.; Negri, T.; Piacenza, C.; Stacchiotti, S.; Gronchi, A.; Pastorino, U.; Pierotti, M.A.; et al. Analysis of potential receptor tyrosine kinase targets in intimal and mural sarcomas. J. Pathol. 2007, 212, 227–235. [Google Scholar] [CrossRef]
- Tawbi, H.; Thomas, D.; Lucas, D.R.; Biermann, J.S.; Schuetze, S.M.; Hart, A.L.; Chugh, R.; Baker, L.H. Epidermal growth factor receptor expression and mutational analysis in synovial sarcomas and malignant peripheral nerve sheath tumors. Oncologist 2008, 13, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.W.; Wang, H.W.; Chen, W.M.; Chao, T.C.; Hsieh, Y.Y.; Hsih, C.H.; Tzeng, C.H.; Chen, P.C.; Yen, C.C. Prevalence and prognostic influence of genomic changes of EGFR pathway markers in synovial sarcoma. J. Surg. Oncol. 2011, 103, 773–781. [Google Scholar] [CrossRef]
- Vesely, K.; Jurajda, M.; Nenutil, R.; Vesela, M. Expression of p53, cyclin D1 and EGFR correlates with histological grade of adult soft tissue sarcomas: A study on tissue microarrays. Neoplasma 2009, 56, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Ghadimi, M.P.; Young, E.D.; Belousov, R.; Zhu, Q.S.; Liu, J.; Lopez, G.; Colombo, C.; Peng, T.; Reynoso, D.; et al. Combining EGFR and mTOR blockade for the treatment of epithelioid sarcoma. Clin. Cancer Res. 2011, 17, 5901–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.L.; Gupta, R.D.; Goldstein, D.; Crowe, P.J. Significance of Phosphorylated Epidermal Growth Factor Receptor and Its Signal Transducers in Human Soft Tissue Sarcoma. Int. J. Mol. Sci. 2017, 18, 1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.L.; Hannan, M.T.; Russell, P.J.; Crowe, P.J. Expression of HER1/EGFR protein in human soft tissue sarcomas. Eur. J. Surg. Oncol. 2006, 32, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; de Arruda, J.A.A.; Arantes, D.A.C.; Costa, S.F.S.; Souza, L.L.; Pontes, H.A.R.; Fonseca, F.P.; Mesquita, R.A.; Nonaka, C.F.W.; Mendonça, E.F.; et al. Evaluation of tumor-infiltrating lymphocytes in osteosarcomas of the jaws: A multicenter study. Virchows Arch. 2019, 474, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, G.; Pili, F.; Contini, M.; De Miglio, M.R.; Marras, V.; Santeufemia, D.A.; Cherchi, C.; Dessole, M.; Cherchi, P.L.; Cossu-Rocca, P. Analysis of epidermal growth factor receptor (EGFR) status in endometrial stromal sarcoma. Eur. J. Gynaecol. Oncol. 2012, 33, 629–632. [Google Scholar]
- Backer, M.V.; Levashova, Z.; Patel, V.; Jehning, B.T.; Claffey, K.; Blankenberg, F.G.; Backer, J.M. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat. Med. 2007, 13, 504–509. [Google Scholar] [CrossRef]
- Winkler, A.M.; Rice, P.F.; Weichsel, J.; Watson, J.M.; Backer, M.V.; Backer, J.M.; Barton, J.K. In vivo, dual-modality OCT/LIF imaging using a novel VEGF receptor-targeted NIR fluorescent probe in the AOM-treated mouse model. Mol. Imaging Biol. 2011, 13, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Kakiuchi-Kiyota, S.; Arnold, L.L.; Johansson, S.L.; Wert, D.; Cohen, S.M. Pathogenesis of human hemangiosarcomas and hemangiomas. Hum. Pathol. 2013, 44, 2302–2311. [Google Scholar] [CrossRef]
- Pakos, E.E.; Goussia, A.C.; Tsekeris, P.G.; Papachristou, D.J.; Stefanou, D.; Agnantis, N.J. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005, 25, 3591–3596. [Google Scholar]
- Stacher, E.; Gruber-Mosenbacher, U.; Halbwedl, I.; Dei Tos, A.P.; Cavazza, A.; Papotti, M.; Carvalho, L.; Huber, M.; Ermert, L.; Popper, H.H. The VEGF-system in primary pulmonary angiosarcomas and haemangioendotheliomas: New potential therapeutic targets? Lung Cancer 2009, 65, 49–55. [Google Scholar] [CrossRef]
- Ho, A.L.; Vasudeva, S.D.; Lae, M.; Saito, T.; Barbashina, V.; Antonescu, C.R.; Ladanyi, M.; Schwartz, G.K. PDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: Implications for combination targeted therapy of synovial sarcoma. Cancer Res. 2012, 72, 4515–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Qian, W.; Uckun, F.M.; Zhou, Z.; Wang, L.; Wang, A.; Mao, H.; Yang, L. IGF-1 receptor targeted nanoparticles for image-guided therapy of stroma-rich and drug resistant human cancer. Proc. SPIE Int. Soc. Opt. Eng. 2016, 9836. [Google Scholar] [CrossRef] [Green Version]
- Moroncini, G.; Maccaroni, E.; Fiordoliva, I.; Pellei, C.; Gabrielli, A.; Berardi, R. Developments in the management of advanced soft-tissue sarcoma—Olaratumab in context. Onco. Targets 2018, 11, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camorani, S.; Hill, B.S.; Collina, F.; Gargiulo, S.; Napolitano, M.; Cantile, M.; Di Bonito, M.; Botti, G.; Fedele, M.; Zannetti, A.; et al. Targeted imaging and inhibition of triple-negative breast cancer metastases by a PDGFRbeta aptamer. Theranostics 2018, 8, 5178–5199. [Google Scholar] [CrossRef]
- Adams, S.F.; Hickson, J.A.; Hutto, J.Y.; Montag, A.G.; Lengyel, E.; Yamada, S.D. PDGFR-alpha as a potential therapeutic target in uterine sarcomas. Gynecol. Oncol. 2007, 104, 524–528. [Google Scholar] [CrossRef]
- Liegl, B.; Gully, C.; Reich, O.; Nogales, F.F.; Beham, A.; Regauer, S. Expression of platelet-derived growth factor receptor in low-grade endometrial stromal sarcomas in the absence of activating mutations. Histopathology 2007, 50, 448–452. [Google Scholar] [CrossRef]
- Rossi, G.; Valli, R.; Bertolini, F.; Marchioni, A.; Cavazza, A.; Mucciarini, C.; Migaldi, M.; Federico, M.; Trentini, G.P.; Sgambato, A. PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology 2005, 46, 522–531. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Vlenterie, M.; van der Graaf, W.T.A.; Hillebrandt-Roeffen, M.H.S.; Blackburn, J.; Ma, X.; Chan, H.; Magias, M.C.; van Erp, A.; van Houdt, L.; et al. Phosphoproteomic Profiling Reveals ALK and MET as Novel Actionable Targets across Synovial Sarcoma Subtypes. Cancer Res. 2017, 77, 4279–4292. [Google Scholar] [CrossRef] [Green Version]
- Roland, C.L.; May, C.D.; Watson, K.L.; Al Sannaa, G.A.; Dineen, S.P.; Feig, R.; Landers, S.; Ingram, D.R.; Wang, W.L.; Guadagnolo, B.A.; et al. Analysis of Clinical and Molecular Factors Impacting Oncologic Outcomes in Undifferentiated Pleomorphic Sarcoma. Ann. Surg. Oncol. 2016, 23, 2220–2228. [Google Scholar] [CrossRef] [Green Version]
- Hiraki-Hotokebuchi, Y.; Yamada, Y.; Kohashi, K.; Yamamoto, H.; Endo, M.; Setsu, N.; Yuki, K.; Ito, T.; Iwamoto, Y.; Furue, M.; et al. Alteration of PDGFRbeta-Akt-mTOR pathway signaling in fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Hum. Pathol. 2017, 67, 60–68. [Google Scholar] [CrossRef]
- Lopez-Guerrero, J.A.; Navarro, S.; Noguera, R.; Carda, C.; Farinas, S.C.; Pellin, A.; Llombart-Bosch, A. Mutational analysis of the c-KIT AND PDGFRalpha in a series of molecularly well-characterized synovial sarcomas. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 2005, 14, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Sieber, T.; Schoeler, D.; Ringel, F.; Pascu, M.; Schriever, F. Selective internalization of monoclonal antibodies by B-cell chronic lymphocytic leukaemia cells. Br. J. Haematol. 2003, 121, 458–461. [Google Scholar] [CrossRef]
- Klohs, J.; Grafe, M.; Graf, K.; Steinbrink, J.; Dietrich, T.; Stibenz, D.; Bahmani, P.; Kronenberg, G.; Harms, C.; Endres, M.; et al. In vivo imaging of the inflammatory receptor CD40 after cerebral ischemia using a fluorescent antibody. Stroke 2008, 39, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.M.; Biddolph, S.; Lucas, S.B.; Howells, D.D.; Picton, S.; McGee, J.O.; O’Leary, J.J. CD40 upregulation is independent of HHV-8 in the pathogenesis of Kaposi’s sarcoma. Mol. Pathol. 1999, 52, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechtersheimer, G.; Barth, T.; Ludwig, R.; Staudter, M.; Moller, P. Differential expression of leukocyte differentiation antigens in small round blue cell sarcomas. Cancer 1993, 71, 237–248. [Google Scholar] [CrossRef]
- Ottaiano, A.; De Chiara, A.; Perrone, F.; Botti, G.; Fazioli, F.; De Rosa, V.; Mozzillo, N.; Ravo, V.; Morrica, B.; Gallo, C.; et al. Prognostic value of CD40 in adult soft tissue sarcomas. Clin. Cancer Res. 2004, 10, 2824–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammer, J.; Plettenberg, A.; Weninger, W.; Diller, B.; Mildner, M.; Uthman, A.; Issing, W.; Sturzl, M.; Tschachler, E. CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi’s sarcoma. Am. J. Pathol. 1996, 148, 1387–1396. [Google Scholar]
- Kalim, M.; Wang, S.; Liang, K.; Khan, M.S.I.; Zhan, J. Engineered scPDL1-DM1 drug conjugate with improved in vitro analysis to target PD-L1 positive cancer cells and intracellular trafficking studies in cancer therapy. Genet. Mol. Biol. 2020, 42, e20180391. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, H.; Zhang, R.; Jiang, H.; Xu, H.; Pan, W.; Gao, X.; Sun, Z. Near-infrared fluorescence-labeled anti-PD-L1-mAb for tumor imaging in human colorectal cancer xenografted mice. J. Cell. Biochem. 2019, 120, 10239–10247. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrych, A.; Pęksa, R.; Kunc, M.; Krawczyk, M.; Izycka-Swieszewska, E.; Biernat, W.; Bień, E. The PD-L1/PD-1 axis expression on tumor-infiltrating immune cells and tumor cells in pediatric rhabdomyosarcoma. Pathol. Res. Pract. 2019, 215, 152700. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Kawamura, T.; Riddell, M.; Hikita, T.; Yanagi, T.; Umemura, H.; Nakayama, M. Regulation of programmed cell death ligand 1 expression by atypical protein kinase C lambda/iota in cutaneous angiosarcoma. Cancer Sci. 2019, 110, 1780–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kim, M.W.; Park, D.Y. Indirect ultrasound guidance increased accuracy of the glenohumeral injection using the superior approach: A cadaveric study of injection accuracy. Ann. Rehabil. Med. 2013, 37, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Mauch, C.; Wagener-Ryczek, S.; Schoemmel, M.; Buettner, R.; Quaas, A.; Helbig, D. Immune-phenotyping of pleomorphic dermal sarcomas suggests this entity as a potential candidate for immunotherapy. Cancer Immunol. Immunother. 2019, 68, 973–982. [Google Scholar] [CrossRef]
- Kosemehmetoglu, K.; Ozogul, E.; Babaoglu, B.; Tezel, G.G.; Gedikoglu, G. Programmed Death Ligand 1 (PD-L1) Expression in Malignant Mesenchymal Tumors. Turk. Patoloji. Derg. 2017, 1, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Orth, M.F.; Buecklein, V.L.; Kampmann, E.; Subklewe, M.; Noessner, E.; Cidre-Aranaz, F.; Romero-Pérez, L.; Wehweck, F.S.; Lindner, L.; Issels, R.; et al. A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol. Immunother. 2020, 69, 1353–1362. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, M.; Sung, M.; Lee, S.E.; Kim, Y.J.; Choi, Y.L. Status of programmed death-ligand 1 expression in sarcomas. J. Transl. Med. 2018, 16, 303. [Google Scholar] [CrossRef]
- Paydas, S.; Bagir, E.K.; Deveci, M.A.; Gonlusen, G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016, 33, 93. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; He, Q.; Yearley, J.H.; Emerson, R.; Vignali, M.; Zhang, Y.; Redman, M.W.; Baker, K.K.; Cooper, S.; Donahue, B.; et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017, 123, 3291–3304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanes, E.D.; Friedman, L.A.; Mills, A.M. PD-L1 Expression and Tumor-infiltrating Lymphocytes in Uterine Smooth Muscle Tumors: Implications for Immunotherapy. Am. J. Surg. Pathol. 2019, 43, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Amaya, C.N.; Wians, F.H., Jr.; Bryan, B.A. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology 2017, 49, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.C.; Maclean, F.M.; Sioson, L.; Tran, D.; Bonar, F.; Mahar, A.; Cheah, A.L.; Russell, P.; Grimison, P.; Richardson, L.; et al. Prevalence of PD-L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS ONE 2020, 15, e0222551. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Wang, Z.; Cui, C.; Guan, X.; Dong, B.; Zhao, M.; Wu, J.; Tian, X.; Hao, C. Comprehensive immune characterization and T-cell receptor repertoire heterogeneity of retroperitoneal liposarcoma. Cancer Sci. 2019, 110, 3038–3048. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Wang, J.; Cai, W.; Lao, I.; Shi, Y.; Luo, X.; Yan, W. Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas. Ann. Transl. Med. 2019, 7, 387. [Google Scholar] [CrossRef]
- Edris, B.; Espinosa, I.; Muhlenberg, T.; Mikels, A.; Lee, C.H.; Steigen, S.E.; Zhu, S.; Montgomery, K.D.; Lazar, A.J.; Lev, D.; et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J. Pathol. 2012, 227, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Ehlerding, E.B.; England, C.G.; Majewski, R.L.; Valdovinos, H.F.; Jiang, D.; Liu, G.; McNeel, D.G.; Nickles, R.J.; Cai, W. ImmunoPET Imaging of CTLA-4 Expression in Mouse Models of Non-small Cell Lung Cancer. Mol. Pharm. 2017, 14, 1782–1789. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.K.; Lee, Y.C.; Cheng, T.L.; Lai, C.H.; Hsu, C.K.; Kuo, C.H.; Hsu, Y.Y.; Li, J.T.; Chang, B.I.; Ma, C.Y.; et al. Tumor Endothelial Marker 1 (TEM1/Endosialin/CD248) Enhances Wound Healing by Interacting with Platelet-Derived Growth Factor Receptors. J. Investig. Derm. 2019, 139, 2204–2214.e7. [Google Scholar] [CrossRef]
- Naylor, A.J.; McGettrick, H.M.; Maynard, W.D.; May, P.; Barone, F.; Croft, A.P.; Egginton, S.; Buckley, C.D. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS ONE 2014, 9, e107146. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget 2019, 10, 993–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzyk, Ł. Biomarkers Discovery for Colorectal Cancer: A Review on Tumor Endothelial Markers as Perspective Candidates. Dis. Markers 2016, 2016, 4912405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouleau, C.; Smale, R.; Fu, Y.S.; Hui, G.; Wang, F.; Hutto, E.; Fogle, R.; Jones, C.M.; Krumbholz, R.; Roth, S.; et al. Endosialin is expressed in high grade and advanced sarcomas: Evidence from clinical specimens and preclinical modeling. Int. J. Oncol. 2011, 39, 73–89. [Google Scholar] [CrossRef]
- Palmerini, E.; Benassi, M.S.; Quattrini, I.; Pazzaglia, L.; Donati, D.; Benini, S.; Gamberi, G.; Gambarotti, M.; Picci, P.; Ferrari, S. Prognostic and predictive role of CXCR4, IGF-1R and Ezrin expression in localized synovial sarcoma: Is chemotaxis important to tumor response? Orphanet. J. Rare. Dis. 2015, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Ptaszyński, K.; Szumera-Ciećkiewicz, A.; Zakrzewska, K.; Tuziak, T.; Mrozkowiak, A.; Rutkowski, P. Her2, EGFR and TOPIIA gene amplification and protein expression in synovial sarcoma before and after combined treatment. Pol. J. Pathol. 2009, 60, 10–18. [Google Scholar]
- Ahlen, J.; Wejde, J.; Brosjo, O.; von Rosen, A.; Weng, W.H.; Girnita, L.; Larsson, O.; Larsson, C. Insulin-like growth factor type 1 receptor expression correlates to good prognosis in highly malignant soft tissue sarcoma. Clin. Cancer Res. 2005, 11, 206–216. [Google Scholar]
- Van der Ven, L.T.; Roholl, P.J.; Gloudemans, T.; Van Buul-Offers, S.C.; Welters, M.J.; Bladergroen, B.A.; Faber, J.A.; Sussenbach, J.S.; Den Otter, W. Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br. J. Cancer 1997, 75, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.L.; Chawla, S.P.; Attia, S.; Schöffski, P.; Gelderblom, H.; Chmielowski, B.; Le Cesne, A.; Van Tine, B.A.; Trent, J.C.; Patel, S.; et al. A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas. Cancer 2019, 125, 2445–2454. [Google Scholar] [CrossRef] [Green Version]
- Kilvaer, T.K.; Valkov, A.; Sorbye, S.; Smeland, E.; Bremnes, R.M.; Busund, L.T.; Donnem, T. Profiling of VEGFs and VEGFRs as prognostic factors in soft tissue sarcoma: VEGFR-3 is an independent predictor of poor prognosis. PLoS ONE 2010, 5, e15368. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, P.P.; Musi, E.; Schwartz, G.K. Preclinical Evaluation of Nintedanib, a Triple Angiokinase Inhibitor, in Soft-tissue Sarcoma: Potential Therapeutic Implication for Synovial Sarcoma. Mol. Cancer 2018, 17, 2329–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinicaltrials.gov Ramucirumab. Available online: https://clinicaltrials.gov/ct2/show/NCT04145700?term=ramucirumab&cond=Soft+Tissue+Sarcoma&draw=2&rank=1 (accessed on 22 June 2020).
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet. Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Mitsiades, N.; Yu, W.H.; Poulaki, V.; Tsokos, M.; Stamenkovic, I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001, 61, 577–581. [Google Scholar] [PubMed]
- Steinkamp, P.J.; Pranger, B.K.; Li, M.; Linssen, M.D.; Voskuil, F.J.; Been, L.B.; van Leeuwen, B.L.; Suurmeijer, A.J.H.; Nagengast, W.B.; Kruijff, S.K.; et al. Fluorescence-guided visualization of soft tissue sarcomas by targeting vascular endothelial growth factor-A: A phase 1 single-center clinical trial. J. Nucl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; Terwisscha van Scheltinga, A.G.T.; Jansen, L.; de Vries, J.; Lub-de Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef]
- Pellat, A.; Vaquero, J.; Fouassier, L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology 2018, 67, 762–773. [Google Scholar] [CrossRef]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: Recent advances. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Li, C.W.; Zhao, L.; Subramaniam, S.; Yu, X.M.; Li, Y.Y.; de Chen, H.; Li, T.Y.; Shen, L.; Shi, L.; et al. Differential Expression Patterns of EGF, EGFR, and ERBB4 in Nasal Polyp Epithelium. PLoS ONE 2016, 11, e0156949. [Google Scholar] [CrossRef]
- Huisman, B.W.; Burggraaf, J.; Vahrmeijer, A.L.; Schoones, J.W.; Rissmann, R.A.; Sier, C.F.M.; van Poelgeest, M.I.E. Potential targets for tumor-specific imaging of vulvar squamous cell carcinoma: A systematic review of candidate biomarkers. Gynecol. Oncol. 2020, 156, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. Biomed. Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov Cetuximab. Available online: https://clinicaltrials.gov/ct2/show/NCT00148109?term=cetuximab&cond=Soft+Tissue+Sarcoma&draw=3&rank=1 (accessed on 22 June 2020).
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neurooncol. 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer. Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [Green Version]
- Colman, R.W.; Pixley, R.A.; Sainz, I.M.; Song, J.S.; Isordia-Salas, I.; Muhamed, S.N.; Powell, J.A., Jr.; Mousa, S.A. Inhibition of angiogenesis by antibody blocking the action of proangiogenic high-molecular-weight kininogen. J. Thromb. Haemost. 2003, 1, 164–170. [Google Scholar] [CrossRef]
- Samkoe, K.S.; Gunn, J.R.; Marra, K.; Hull, S.M.; Moodie, K.L.; Feldwisch, J.; Strong, T.V.; Draney, D.R.; Hoopes, P.J.; Roberts, D.W.; et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: A Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use. Mol. Imaging Biol. 2017, 19, 512–521. [Google Scholar] [CrossRef]
- Clinicaltrials.gov Panitumumab (Head&Neck Cancer). Available online: https://clinicaltrials.gov/ct2/show/NCT03405142?term=Panitumumab-IRDye800&draw=2&rank=4 (accessed on 22 June 2020).
- Clinicaltrials.gov Panitumumab (Lung Cancer). Available online: https://clinicaltrials.gov/ct2/show/NCT03582124?term=Panitumumab-IRDye800&draw=2&rank=3 (accessed on 22 June 2020).
- Clinicaltrials.gov Panitumumab. Available online: https://clinicaltrials.gov/ct2/show/NCT02415881?term=Panitumumab-IRDye800&draw=2&rank=2 (accessed on 22 June 2020).
- Friedrichs, N.; Kuchler, J.; Endl, E.; Koch, A.; Czerwitzki, J.; Wurst, P.; Metzger, D.; Schulte, J.H.; Holst, M.I.; Heukamp, L.C.; et al. Insulin-like growth factor-1 receptor acts as a growth regulator in synovial sarcoma. J. Pathol. 2008, 216, 428–439. [Google Scholar] [CrossRef]
- Xie, Y.; Skytting, B.; Nilsson, G.; Brodin, B.; Larsson, O. Expression of insulin-like growth factor-1 receptor in synovial sarcoma: Association with an aggressive phenotype. Cancer Res. 1999, 59, 3588–3591. [Google Scholar]
- Clinicaltrials.gov Ganitumab. Available online: https://clinicaltrials.gov/ct2/show/NCT00819169?term=Ganitumab&cond=Soft+Tissue+Sarcoma&draw=2&rank=8 (accessed on 22 June 2020).
- Clinicaltrials.gov AMG-479. Available online: https://clinicaltrials.gov/ct2/show/NCT00562380?term=Ganitumab&cond=Soft+Tissue+Sarcoma&draw=2&rank=6 (accessed on 22 June 2020).
- Olmos, D.; Postel-Vinay, S.; Molife, L.R.; Okuno, S.H.; Schuetze, S.M.; Paccagnella, M.L.; Batzel, G.N.; Yin, D.; Pritchard-Jones, K.; Judson, I.; et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: A phase 1 expansion cohort study. Lancet. Oncol. 2010, 11, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Pappo, A.S.; Vassal, G.; Crowley, J.J.; Bolejack, V.; Hogendoorn, P.C.; Chugh, R.; Ladanyi, M.; Grippo, J.F.; Dall, G.; Staddon, A.P.; et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: Results of a Sarcoma Alliance for Research Through Collaboration study. Cancer 2014, 120, 2448–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoffski, P.; Adkins, D.; Blay, J.Y.; Gil, T.; Elias, A.D.; Rutkowski, P.; Pennock, G.K.; Youssoufian, H.; Gelderblom, H.; Willey, R.; et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur. J. Cancer 2013, 49, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zeng, X.; Li, Q.; Gaillard-Kelly, M.; Wagner, C.R.; Yee, D. Fluorescent tumour imaging of type I IGF receptor in vivo: Comparison of antibody-conjugated quantum dots and small-molecule fluorophore. Br. J. Cancer 2009, 101, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.T.; Chao, H.W.; Lai, A.C.; Lin, S.H.; Chang, Y.J.; Huang, Y.S. CPEB2-activated PDGFRalpha mRNA translation contributes to myofibroblast proliferation and pulmonary alveologenesis. J. Biomed. Sci. 2020, 27, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.H.; Lin, J.S.; Yang, C.C.; Cheng, H.W.; Chang, K.W.; Liu, C.J. Overexpression of Platelet-Derived Growth Factor and Its Receptor Are Correlated with Oral Tumorigenesis and Poor Prognosis in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 2360. [Google Scholar] [CrossRef] [Green Version]
- Santilli, F.; Basili, S.; Ferroni, P.; Davi, G. CD40/CD40L system and vascular disease. Intern. Emerg. Med. 2007, 2, 256–268. [Google Scholar] [CrossRef]
- Vonderheide, R.H. Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 2007, 13, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Elmetwali, T.; Young, L.S.; Palmer, D.H. Fas-associated factor (Faf1) is a novel CD40 interactor that regulates CD40-induced NF-kappaB activation via a negative feedback loop. Cell Death Dis. 2014, 5, e1213. [Google Scholar] [CrossRef] [Green Version]
- Gennatas, S.; Chamberlain, F.; Carter, T.; Slater, S.; Cojocaru, E.; Lambourn, B.; Stansfeld, A.; Todd, R.; Verrill, M.; Ali, N.; et al. Real-world experience with doxorubicin and olaratumab in soft tissue sarcomas in England and Northern Ireland. Clin. Sarcoma Res. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Piechutta, M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). Esmo Open 2019, 4, e000510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinicaltrials.gov APX005M. Available online: https://clinicaltrials.gov/ct2/show/NCT03719430?term=APX005M&cond=Soft+Tissue+Sarcoma&draw=2&rank=1 (accessed on 22 June 2020).
- Gu, L.; Ruff, L.E.; Qin, Z.; Corr, M.; Hedrick, S.M.; Sailor, M.J. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv. Mater. 2012, 24, 3981–3987. [Google Scholar] [CrossRef] [Green Version]
- Tap, W.D.; Wagner, A.J.; Schöffski, P.; Martin-Broto, J.; Krarup-Hansen, A.; Ganjoo, K.N.; Yen, C.C.; Abdul Razak, A.R.; Spira, A.; Kawai, A.; et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 2020, 323, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Kodaira, M.; Satoh, T.; Kudo, T.; Takahashi, S.; Nakano, K.; Ando, Y.; Shimokata, T.; Mori, J.; Inoue, K.; et al. Phase 1 study of olaratumab plus doxorubicin in Japanese patients with advanced soft-tissue sarcoma. Cancer Sci. 2018, 109, 3962–3970. [Google Scholar] [CrossRef]
- Wagner, A.J.; Kindler, H.; Gelderblom, H.; Schoffski, P.; Bauer, S.; Hohenberger, P.; Kopp, H.G.; Lopez-Martin, J.A.; Peeters, M.; Reichardt, P.; et al. A phase II study of a human anti-PDGFRalpha monoclonal antibody (olaratumab, IMC-3G3) in previously treated patients with metastatic gastrointestinal stromal tumors. Ann. Oncol. 2017, 28, 541–546. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Tap, W.D.; Qin, L.X.; Livingston, M.B.; Undevia, S.D.; Chmielowski, B.; Agulnik, M.; Schuetze, S.M.; Reed, D.R.; Okuno, S.H.; et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: A multicentre, open-label, phase 2 trial. Lancet. Oncol. 2013, 14, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, K.M.; Chang, K.W.; Truong, M.T.; Kwok, S.; West, R.B.; Heerema-McKenney, A.E. β-Adrenergic receptor expression in vascular tumors. Mod. Pathol. 2012, 25, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Federico, S.M.; Caldwell, K.J.; McCarville, M.B.; Daryani, V.M.; Stewart, C.F.; Mao, S.; Wu, J.; Davidoff, A.M.; Santana, V.M.; Furman, W.L.; et al. Phase I expansion cohort to evaluate the combination of bevacizumab, sorafenib and low-dose cyclophosphamide in children and young adults with refractory or recurrent solid tumours. Eur. J. Cancer 2020, 132, 35–42. [Google Scholar] [CrossRef]
- Hong, D.S.; Garrido-Laguna, I.; Ekmekcioglu, S.; Falchook, G.S.; Naing, A.; Wheler, J.J.; Fu, S.; Moulder, S.L.; Piha-Paul, S.; Tsimberidou, A.M.; et al. Dual inhibition of the vascular endothelial growth factor pathway: A phase 1 trial evaluating bevacizumab and AZD2171 (cediranib) in patients with advanced solid tumors. Cancer 2014, 120, 2164–2173. [Google Scholar] [CrossRef] [Green Version]
- Tap, W.D.; Federman, N.; Eilber, F.C. Targeted therapies for soft-tissue sarcomas. Expert Rev. Anticancer 2007, 7, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.S.; Tice, D.A.; Soria, J.C. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrus, P.; Fernandez, T.L.; Kwon, M.M.; Huang, J.L.; Lei, V.; Safikhan, N.S.; Karunakaran, S.; O’Shannessy, D.J.; Zheng, X.; Catrina, S.B.; et al. Specific loss of adipocyte CD248 improves metabolic health via reduced white adipose tissue hypoxia, fibrosis and inflammation. EBioMedicine 2019, 44, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodanovich, D.A.; Choong, P.F.M. Soft-tissue Sarcomas. Indian J. Orthop. 2018, 52, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: http://www.proteinatlas.org (accessed on 13 December 2020).
- Grothey, A.; Strosberg, J.R.; Renfro, L.A.; Hurwitz, H.I.; Marshall, J.L.; Safran, H.; Guarino, M.J.; Kim, G.P.; Hecht, J.R.; Weil, S.C.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Monotherapy Ontuxizumab (MORAb-004) Plus Best Supportive Care in Patients with Chemorefractory Metastatic Colorectal Cancer. Clin. Cancer Res. 2018, 24, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Kersting, C.; Packeisen, J.; Leidinger, B.; Brandt, B.; von Wasielewski, R.; Winkelmann, W.; van Diest, P.J.; Gosheger, G.; Buerger, H. Pitfalls in immunohistochemical assessment of EGFR expression in soft tissue sarcomas. J. Clin. Pathol. 2006, 59, 585–590. [Google Scholar] [CrossRef]

| Score | 0 | 1 | 2 |
|---|---|---|---|
| Sample size | 0–100 | 101–500 | >500 |
| Percentage of positive STS samples | 0–33% | 33–67% | >67% |
| Pattern of expression * | Focal | Heterogeneous | Diffuse |
| Internalization | not described | Yes | |
| Previously imaged | not described | Yes, but not with NIRF imaging | Yes, NIRF imaging |
| Biomarker | Therapeutic Antibody | N | % Positive STS (Mean% + Range) | Pattern of Expression | Internalization | Previously Imaged | Score | Literature |
|---|---|---|---|---|---|---|---|---|
| Tumor endothelial marker 1 (TEM1/ endosialin/ CD248) | Ontuxizumab (MORAb-004) | 768 | 77% (55–100) | Diffuse | Yes, [25] | NIRF imaging [26] | 9 | [26,27,28,29] |
| Vascular endothelial growth factor receptor-1 (VEGFR-1) | Aflibercept Bevacizumab | 477 | 76% (22–100) | Diffuse | Yes, [30] | NIRF imaging [30,31] | 8 | [32,33,34,35,36,37,38,39] |
| Epidermal growth factor receptor (EGFR) | Cetuximab | 1918 | 53% (0–100) | Diffuse | Yes, [40] | NIRF imaging [41] | 8 | [27,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] |
| Vascular endothelial growth factor receptor-2 (VEGFR-2) | Aflibercept Bevacizumab Ramucirumab | 449 | 71% (11–100) | Diffuse | Yes, [77] | NIRF imaging [78] | 7 | [33,34,35,36,38,39,79,80,81] |
| Insulin-like growth factor 1 receptor (IGF-1R) | Ganitumab (AMG 479) Teprotumumab Cixutumumab Figitumumab | 507 | 63% (25–100) | Diffuse | Yes, [82] | NIRF imaging [83] | 7 | [63,64,82,84,85,86,87,88,89] |
| Platelet derived growth factor receptor α (PDGFRα) | Olaratumab | 1536 | 64% (0–100) | Diffuse | Yes, [84] | NIRF imaging [85] | 7 | [27,34,36,38,42,43,44,45,46,47,48,49,51,82,86,87,88,89,90,91,92] |
| Cluster of differentiation 40 (CD40) | APX005M | 153 | 62% (17–86) | Diffuse | Yes, [93] | NIRF imaging [94] | 7 | [95,96,97,98] |
| Programmed death-ligand 1 (PD-L1/CD 274/B7-H1) | Atezolizumab Avelumab Durvalumab Envafolimab | 1492 | 31% (0–76) | Heterogeneous (focal and diffuse) | Yes, [99] | NIRF imaging [100] | 6 | [101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
| Leucine-rich repeat containing 15 (LRRC15) | ABBV-085 | 635 | 40% | Diffuse | Not described | Not described | 4 | [102] |
| Receptor tyrosine kinase-like orphan receptor 2 (ROR2) | CAB-ROR2-ADC | 237 | 72% | Not described | Not described | Not described | 3 | [119] |
| Cytotoxic T-Lymphocyte-associated protein 4 (CTLA-4/CD152) | Ipilimumab Tremelimumab | 10 | 30% | Not described | Yes, [120] | Not with NIRF imaging [120] | 2 | [59] |
| Biomarker | N | Positive Tumors Mean% (Range) | Expression Pattern | Present after RTx | Literature |
|---|---|---|---|---|---|
| Myxofibrosarcoma | |||||
| TEM1 | 34 | 100 (100) | Diffuse | Yes, [27] | [27] |
| EGFR | 97 | 38 (0–89) | Heterogeneous | Yes, [27] | [26,53,65] |
| PDGFRα | 34 | 77 (77) | Not described | Yes, [27] | [27] |
| Undifferentiated soft tissue sarcoma | |||||
| TEM1 | 128 | 81 (73–89) | Diffuse | N.D. | [28,29] |
| VEGFR-1 | 81 | 68 (68) | Not described | N.D. | [36] |
| EGFR | 287 | 62 (5–95) | Heterogeneous | N.D. | [50,57,65,70] |
| VEGFR-2 | 81 | 6 (6) | Not described | N.D. | [36] |
| IGF-1R | 120 | 25 (25) | Not described | N.D. | [90] |
| PDGFRα | 432 | 79 (63–99) | Diffuse | N.D. | [35,50,126] |
| Synovial sarcoma | |||||
| TEM1 | 70 | 71 (62–80) | Heterogeneous | N.D. | [28,29] |
| VEGFR-1 | 27 | 70 (70) | Not described | N.D. | [27] |
| EGFR | 160 | 86 (71–100) | Heterogeneous | Yes, [127] | [52,58,66,69,70,71] |
| VEGFR-2 | 27 | 4 (4) | Not described | N.D. | [27] |
| IGF-1R | 195 | 57 (35–80) | Not described | N.D. | [81,82,128,129] |
| PDGFRα | 136 | 69 (44–84) | Not described | N.D. | [35,81,88,91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijs, Z.; Shifai, A.N.; Bosma, S.E.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Keereweer, S.; Bovée, J.V.M.G.; van de Sande, M.A.J.; Sier, C.F.M.; van Driel, P.B.A.A. Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review. Cancers 2021, 13, 557. https://doi.org/10.3390/cancers13030557
Rijs Z, Shifai AN, Bosma SE, Kuppen PJK, Vahrmeijer AL, Keereweer S, Bovée JVMG, van de Sande MAJ, Sier CFM, van Driel PBAA. Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review. Cancers. 2021; 13(3):557. https://doi.org/10.3390/cancers13030557
Chicago/Turabian StyleRijs, Zeger, A. Naweed Shifai, Sarah E. Bosma, Peter J. K. Kuppen, Alexander L. Vahrmeijer, Stijn Keereweer, Judith V. M. G. Bovée, Michiel A. J. van de Sande, Cornelis F. M. Sier, and Pieter B. A. A. van Driel. 2021. "Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review" Cancers 13, no. 3: 557. https://doi.org/10.3390/cancers13030557
APA StyleRijs, Z., Shifai, A. N., Bosma, S. E., Kuppen, P. J. K., Vahrmeijer, A. L., Keereweer, S., Bovée, J. V. M. G., van de Sande, M. A. J., Sier, C. F. M., & van Driel, P. B. A. A. (2021). Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review. Cancers, 13(3), 557. https://doi.org/10.3390/cancers13030557