Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances
Abstract
:Simple Summary
Abstract
1. Introduction
2. Synthesis of Melanin in Mammals
3. Synthesis of Melanin-Like Nanoparticles
4. Melanin and Its Putative Role in Immunity
- (a)
- Alpha melanocyte-stimulating hormone (α-MSH), an endogenous peptide hormone of the melanocortin family, plays a key role in melanogenesis through binding to its receptor MC1R (melanocortin 1 receptor). It appears that α-MSH has a wide range of activities that include anti-inflammatory effects and immunomodulation on macrophages and neutrophils [14]. These anti-inflammatory effects have been well established in several models of inflammatory or ischemia/reperfusion injury and bacterial endotoxin-induced inflammation [15]. In addition, α-MSH can promote regulatory T-lymphocytes (Treg), which modulate immunity-targeting specific antigens [16].
- (b)
- Melanogenesis plays a role in invertebrate innate immunity. Insects commonly activate the formation of melanin around intruding microorganisms in a process known as melanization [17]. Within minutes after infection, microbes are encapsulated within melanin and the generation of free radical byproducts during the formation of this capsule is thought to aid in killing them. This process constitutes a major aspect of the innate immune defense system against invading pathogens in invertebrates but does not occur in mammals. The situation might even be the opposite in mammals, as melanin synthesis by Cryptococcus neoformans actually increases its virulence by protecting the fungus against phagocytic killing by the host [18]. Moreover, different strains of mice that differed only in the gene encoding tyrosinase, a key enzyme in the synthesis of melanin, showed no difference in the clinical course of malaria infection [19]. Finally, synthetic melanin suppresses cytokine production in macrophages stimulated with lipopolysaccharide [20].
- (c)
- The dendritic nature of melanocytes and their strategic location in the skin raised the idea that they could play a role in adaptive immunity to external pathogens [21]. Melanocytes indeed exhibit phagocytic functions, and phagosomes are transported from the cell surface to the melanosomes that contain many lysosomal enzymes [22,23]. Further studies have shown that melanocytes can act as antigen-presenting cells [22,24]. In addition, in mice with melanocytosis, melanin granules in the skin are continuously captured and transported to regional lymph nodes by Langerhans cells [25,26]. However, in the literature it has not been mentioned that naturally occurring melanin can promote an adaptive immune response such as through antibodies or cytotoxic T-lymphocytes in vivo.
5. Potential Applications of Melanin in Medicine
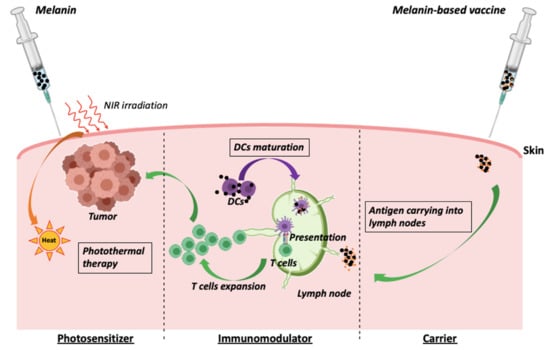
5.1. Melanin as an Adjuvant in Cancer Vaccines
5.2. Melanin as a Photosensitizer in Cancer Photothermal Therapy
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [Green Version]
- Cuzzubbo, S.; Mangsbo, S.; Habra, K.; Nagarajan, D.; Pockley, A.G.; McArdle, S.E. Cancer vaccines: Adjuvant potency, importance of age, lifestyle and treatments. Front. Immunol. 2021, 11, 615240. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Tian, H.; Holmes, C.; Matsunaga, J.; Roffler-Tarlov, S.; Hearing, V.J. Tyrosinase: A developmentally specific major determinant of peripheral dopamine. FASEB J. 2003, 17, 1248–1255. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal. Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment. Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk-Wetula, D.; Wieczorek, J.; Płonka, P.M. Splenic melanosis in agouti and black mice. Acta Biochim. Pol. 2015, 62, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Tsujii, T.; Seno, S. Melano-macrophage centers in the aglomerular kidney of the sea horse (teleosts): Morphologic studies on its formation and possible function. Anat. Rec. 1990, 226, 460–470. [Google Scholar] [CrossRef]
- Yue, Y.; Zhao, X. Melanin-Like Nanomedicine in Photothermal Therapy Applications. Int. J. Mol. Sci. 2021, 22, 399. [Google Scholar] [CrossRef]
- Deng, R.H.; Zou, M.Z.; Zheng, D.; Peng, S.Y.; Liu, W.; Bai, X.F.; Chen, H.S.; Sun, Y.; Zhou, P.H.; Zhang, X.Z. Nanoparticles from Cuttlefish Ink Inhibit Tumor Growth by Synergizing Immunotherapy and Photothermal Therapy. ACS Nano 2019, 13, 8618–8629. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Ge, M.; Iocozzia, J.; Lin, Z.; Zhang, K.Q.; Lai, Y. Immobilization of Pt Nanoparticles via Rapid and Reusable Electropolymerization of Dopamine on TiO2 Nanotube Arrays for Reversible SERS Substrates and Nonenzymatic Glucose Sensors. Small 2017, 13, 1604240. [Google Scholar] [CrossRef]
- Brzoska, T.; Böhm, M.; Lügering, A.; Loser, K.; Luger, T.A. Terminal signal: Anti-inflammatory effects of α-melanocyte-stimulating hormone related peptides beyond the pharmacophore. Adv. Exp. Med. Biol. 2010, 681, 107–116. [Google Scholar]
- Lipton, J.M.; Catania, A. Mechanisms of antiinflammatory action of the neuroimmunomodulatory peptide alpha-MSH. Ann. N. Y. Acad. Sci. 1998, 840, 373–380. [Google Scholar] [CrossRef]
- Taylor, A.W.; Lee, D.J. The alpha-melanocyte stimulating hormone induces conversion of effector T cells into treg cells. J. Transplant 2011, 2011, 246856. [Google Scholar] [CrossRef] [Green Version]
- Viljakainen, L. Evolutionary genetics of insect innate immunity. Brief. Funct. Genom. 2015, 14, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Rosas, A.L.; Nosanchuk, J.D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2000, 3, 354–358. [Google Scholar] [CrossRef]
- Waisberg, M.; Vickers, B.K.; Yager, S.B.; Lin, C.K.; Pierce, S.K. Testing in mice the hypothesis that melanin is protective in malaria infections. PLoS ONE 2012, 7, e29493. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tusé, D. Synthetic melanin suppresses production of proinflammatory cytokines. Cell Immunol. 2000, 199, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.; et al. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Poole, I.C.; Mutis, T.; van den Wijngaard, R.M.; Westerhof, W.; Ottenhoff, T.; de Vries, R.R.; Das, P.K. A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J. Immunol. 1993, 151, 7284–7292. [Google Scholar] [PubMed]
- Diment, S.; Eidelman, M.; Rodriguez, G.M.; Orlow, S.J. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J. Biol. Chem. 1995, 270, 4213–4215. [Google Scholar] [CrossRef] [Green Version]
- Gasque, P.; Jaffar-Bandjee, M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Yoshino, M.; Yamazaki, H.; Naito, M.; Iyoda, T.; Omatsu, Y.; Shimoyama, S.; Letterio, J.J.; Nakabayashi, T.; Tagaya, H.; et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 2001, 13, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, M.; Yamazaki, H.; Shultz, L.D.; Hayashi, S. Constant rate of steady-state self-antigen trafficking from skin to regional lymph nodes. Int. Immunol. 2006, 18, 1541–1548. [Google Scholar] [CrossRef]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Yan, Y.; Such, G.K.; Liang, K.; Ochs, C.J.; Postma, A.; Caruso, F. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules. Biomacromolecules 2012, 13, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Chen, T.; Liu, H.; Su, S. Preparation and adsorption performance research of large-volume hollow mesoporous polydopamine microcapsules. MRS Commun. 2019, 9, 744–749. [Google Scholar] [CrossRef]
- Carpentier, A.F.; Geinguenaud, F.; Tran, T.; Sejalon, F.; Martin, A.; Motte, L.; Tartour, E.; Banissi, C. Synthetic melanin bound to subunit vaccine antigens significantly enhances CD8+ T-cell responses. PLoS ONE 2017, 12, e0181403. [Google Scholar] [CrossRef] [Green Version]
- Shamoto, M.; Osada, A.; Shinzato, M.; Kaneko, C.; Yoshida, A. Do epidermal Langerhans cells, migrating from skin lesions, induce the paracortical hyperplasia of dermatopathic lymphadenopathy? Pathol. Int. 1996, 46, 348–354. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Chen, K.; Zhao, Y.; Ma, X.; Wang, L. Doxorubicin-loaded melanin particles for enhanced chemotherapy in drug-resistant anaplastic thyroid cancer cells. J. Nanomater. 2018, 2018, 2603712. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Duan, Y.; Duan, Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control Release 2018, 290, 56–74. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- Li, W.Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.P.; Cheng, G.; Zheng, S.Y. Mitochondria-Targeting Polydopamine Nanoparticles to Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl Mater Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Pokorski, J.K. Poly(lactic-co-glycolic acid) devices: Production and applications for sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Guo, H. Synergic highly effective photothermal-chemotherapy with platinum prodrug linked melanin-like nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 356–363. [Google Scholar] [CrossRef] [Green Version]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- El-Obeid, A.; Al-Harbi, S.; Al-Jomah, N.; Hassib, A. Herbal melanin modulates tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL−6) and vascular endothelial growth factor (VEGF) production. Phytomedicine 2006, 13, 324–333. [Google Scholar] [CrossRef]
- El-Obeid, A.; Alajmi, H.; Harbi, M.; Yahya, W.B.; Al-Eidi, H.; Alaujan, M.; Haseeb, A.; Trivilegio, T.; Alhallaj, A.; Alghamdi, S.; et al. Distinct anti-proliferative effects of herbal melanin on human acute monocytic leukemia THP−1 cells and embryonic kidney HEK293 cells. BMC Complement. Med. Ther. 2020, 20, 154. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Pan, W.; Li, N.; Tang, B. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chem. Commun. 2020, 56, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Viveiros, R.; Correia, T.R.; Correia, I.J.; Bonifácio, V.D.; Casimiro, T.; Aguiar-Ricardo, A. Natural melanin: A potential pH-responsive drug release device. Int. J. Pharm. 2014, 469, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Moon, H.; Hong, S. Recent advances in melanin-like nanomaterials in biomedical applications: A mini review. Biomater. Res. 2019, 23, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Ai, K.L.; Liu, J.H.; Deng, M.; He, Y.Y.; Lu, L.H. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, S.; Zhang, N.; Wang, Y.; Zhong, J.; Sun, X.; Qi, Y.; Chen, X.; Li, Z.; Li, Y. Tailoring Synthetic Melanin Nanoparticles for Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42671–42679. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jing, T.; Xia, X.; Tang, L.; Huang, Z.; Liu, F.; Wang, Z.; Ran, H.; Li, M.; Xia, J. Melanin-loaded biocompatible photosensitive nanoparticles for controlled drug release in combined photothermal-chemotherapy guided by photoacoustic/ultrasound dual-modality imaging. Biomater. Sci. 2019, 7, 4060–4074. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Banissi, C.; Rouchon, M.S.; Tran, T.; Tanchot, C.; Tartour, E.; Carpentier, A.F. The adjuvant effect of melanin is superior to incomplete Freund’s adjuvant in subunit/peptide vaccines in mice. Cancer Immunol. Immunother. 2020, 69, 2501–2512. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Wang, X.; Tian, X.; Qin, W.; Wang, X.; Liang, J.; Zhang, H.; Leng, X. Polydopamine as the Antigen Delivery Nanocarrier for Enhanced Immune Response in Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2330–2342. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Del Valle, E.M.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef] [Green Version]
- Oberländer, U.; Pletinckx, K.; Döhler, A.; Müller, N.; Lutz, M.B.; Arzberger, T.; Riederer, P.; Gerlach, M.; Koutsilieri, E.; Scheller, C. Neuromelanin is an immune stimulator for dendritic cells in vitro. BMC Neurosci. 2011, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Zecca, L.; Wilms, H.; Geick, S.; Claasen, J.H.; Brandenburg, L.O.; Holzknecht, C.; Panizza, M.L.; Zucca, F.A.; Deuschl, G.; Sievers, J.; et al. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: Implications for Parkinson’s disease. Acta Neuropathol. 2008, 116, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Yamanaka, D.; Ishibashi, K.I.; Adachi, Y.; Ohno, N. Solubilized melanin suppresses macrophage function. FEBS Open Bio. 2019, 9, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Chen, W.; Qin, M.; Chen, X.; Wang, Q.; Zhang, Z.; Sun, X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics 2018, 8, 2229–2241. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, Y.; Li, W.S.; Su, Z.; Fadhil, J.I.; Zhang, C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials 2019, 225, 119515. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, eaan5692. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cong, C.; Li, X.; Zhu, R.; Li, A.; Zhao, S.; Li, X.; Cheng, X.; Yang, M.; Gao, D. Nano-drug System Based on Hierarchical Drug Release for Deep Localized/Systematic Cascade Tumor Therapy Stimulating Antitumor Immune Responses. Theranostics 2019, 9, 2897–2909. [Google Scholar] [CrossRef]





| Nanoparticle | Drug Loading/Releasing Capability | Manufacture | Cost of Production | Biocompatibility | Major Advantages | References |
|---|---|---|---|---|---|---|
| Liposomes | High and versatile drug loading: DNA, mRNA, proteins, peptides, immunostimulating agents | Complex synthesis Unstable product | High | Good biocompatibility, but improvement of the biodistribution in vivo is needed |
| [3,37,38,39] |
| Chitosan | Low loading ratio | Easy synthesis Unstable product | Low | Good |
| [40] |
| Polylactic-co-glycolic acid (PLGA) | Poor loading ratio (<10%) with high burst release Versatility: chemotherapeutics, tumor lysate, DNA, mRNA, proteins, peptides, antibodies, genes | Complex synthesis relative to other nanoparticles Stable product | High | Good |
| [40,41,42] |
| Gold | High loading ratio | Reproducible synthesis, stable product | High | Cytotoxicity has been reported depending on the size and charge of gold nanoparticles |
| [43] |
| Synthetic melanin | High loading rate Versatility: chemotherapeutics, tumor lysate, proteins, peptides | Reproducible synthesis, stable product | Low | Good |
| [11,27,30,31,44] |
| Natural melanin | High loading rate | Poorly defined compounds, obtained by extraction from different sources | Low | Good |
| [12,45,46,47,48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuzzubbo, S.; Carpentier, A.F. Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances. Cancers 2021, 13, 1463. https://doi.org/10.3390/cancers13061463
Cuzzubbo S, Carpentier AF. Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances. Cancers. 2021; 13(6):1463. https://doi.org/10.3390/cancers13061463
Chicago/Turabian StyleCuzzubbo, Stefania, and Antoine F. Carpentier. 2021. "Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances" Cancers 13, no. 6: 1463. https://doi.org/10.3390/cancers13061463
APA StyleCuzzubbo, S., & Carpentier, A. F. (2021). Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances. Cancers, 13(6), 1463. https://doi.org/10.3390/cancers13061463