Comparing Apparent Diffusion Coefficient and FNCLCC Grading to Improve Pretreatment Grading of Soft Tissue Sarcoma—A Translational Feasibility Study on Fusion Imaging
Abstract
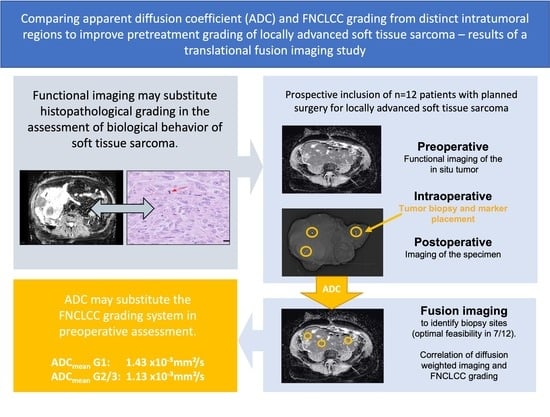
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Magnetic Resonance Imaging
2.3. Intraoperative Core Needle Biopsies
2.4. Computed Tomography Imaging
2.5. Fusion Imaging
2.6. Image Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. FNCLCC Score and Grading
3.3. Fusion Imaging
3.4. Correlation of the ADC and FNCLCC Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3, pp. 2–12. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Coindre, J.-M. Grading of Soft Tissue Sarcomas: Review and Update. Arch. Pathol. Lab. Med. 2006, 130, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Golouh, R.; Bračko, M. What is the current practice in soft tissue sarcoma grading? Radiol. Oncol. 2001, 35, 47–52. [Google Scholar]
- Schneider, N.; Strauss, D.C.; Smith, M.J.; Miah, A.B.; Zaidi, S.; Benson, C.; van Houdt, W.J.; Jones, R.L.; Hayes, A.J.; Fisher, C.; et al. The Adequacy of Core Biopsy in the Assessment of Smooth Muscle Neoplasms of Soft Tissues: Implications for Treatment and Prognosis. Am. J. Surg. Pathol. 2017, 41, 923–931. [Google Scholar] [CrossRef]
- Weigl, H.; Hohenberger, P.; Marx, A.; Vassos, N.; Jakob, J.; Galata, C. Accuracy and Safety of Ultrasound-Guided Core Needle Biopsy of Soft Tissue Tumors in an Outpatient Setting: A Sarcoma Center Analysis of 392 Consecutive Patients. Cancers (Basel) 2021, 13, 5659. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Montesco, M.C.; Coindre, J.M.; Dei Tos, A.P.; Lurkin, A.; Ranchere-Vince, D.; Vecchiato, A.; Decouvelaere, A.V.; Mathoulin-Pelissier, S.; Albert, S.; et al. Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann. Oncol. 2012, 23, 2442–2449. [Google Scholar] [CrossRef]
- Strauss, D.C.; Qureshi, Y.A.; Hayes, A.J.; Thway, K.; Fisher, C.; Thomas, J.M. The role of core needle biopsy in the diagnosis of suspected soft tissue tumours. J. Surg. Oncol. 2010, 102, 523–529. [Google Scholar] [CrossRef]
- Gustafson, P. Soft tissue sarcoma: Epidemiology and prognosis in 508 patients. Acta Orthop. Scand. Suppl. 1994, 259, 2–31. [Google Scholar]
- Nandra, R.; Forsberg, J.; Grimer, R. If your lump is bigger than a golf ball and growing, think Sarcoma. Eur. J. Surg. Oncol. 2015, 41, 1400–1405. [Google Scholar] [CrossRef]
- Crombe, A.; Marcellin, P.J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Gondim Teixeira, P.A.; Gay, F.; Chen, B.; Zins, M.; Sirveaux, F.; Felblinger, J.; Blum, A. Diffusion-weighted magnetic resonance imaging for the initial characterization of non-fatty soft tissue tumors: Correlation between T2 signal intensity and ADC values. Skelet. Radiol. 2016, 45, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Ashikyan, O.; Slepicka, C.; Dettori, N.; Hwang, H.; Callan, A.; Sharma, R.R.; Xi, Y. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: Correlation with histologic grading. Eur. Radiol. 2019, 29, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Birgin, E.; Yang, C.; Hetjens, S.; Reissfelder, C.; Hohenberger, P.; Rahbari, N.N. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: A systematic review and meta-analysis. Cancer 2020, 126, 1917–1928. [Google Scholar] [CrossRef]
- Noebauer-Huhmann, I.M.; Amann, G.; Krssak, M.; Panotopoulos, J.; Szomolanyi, P.; Weber, M.; Czerny, C.; Breitenseher, M.; Grabner, G.; Bogner, W.; et al. Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: Preliminary results. Eur. Radiol. 2015, 25, 2041–2048. [Google Scholar] [CrossRef]
- Mcaddy, N.C.; Hallin, M.; Strauss, D.; Smith, M.; Hayes, A.; Yusuf, S.; Moskovic, E.; Fotiadis, N.; van Houdt, W.; Jones, R.L.; et al. CT imaging improves histopathological grading of retroperitoneal leiomyosarcomas. Eur. J. Surg. Oncol. 2020, 46, 288–292. [Google Scholar] [CrossRef]
- Gondim Teixeira, P.A.; Renaud, A.; Aubert, S.; Ben Haj Amor, M.; Robin, Y.M.; Cotten, A.; Ceugnart, L. Perfusion MR imaging at 3-Tesla: Can it predict tumor grade and histologic necrosis rate of musculoskeletal sarcoma? Diagn. Interv. Imaging 2018, 99, 473–481. [Google Scholar] [CrossRef]
- Reyes Marles, R.H.; Navarro Fernandez, J.L.; Puertas Garcia-Sandoval, J.P.; Santonja Medina, F.; Mohamed Salem, L.; Frutos Esteban, L.; Contreras Gutierrez, J.F.; Castellon Sanchez, M.I.; Ruiz Merino, G.; Claver Valderas, M.A. Clinical value of baseline (18)F-FDG PET/CT in soft tissue sarcomas. Eur. J. Hybrid Imaging 2021, 5, 16. [Google Scholar] [CrossRef]
- Hong, J.H.; Jee, W.H.; Jung, C.K.; Jung, J.Y.; Shin, S.H.; Chung, Y.G. Soft tissue sarcoma: Adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur. Radiol. 2019, 29, 2589–2597. [Google Scholar] [CrossRef]
- Pekcevik, Y.; Kahya, M.O.; Kaya, A. Characterization of Soft Tissue Tumors by Diffusion-Weighted Imaging. Iran. J. Radiol. 2015, 12, e15478. [Google Scholar] [CrossRef]
- Rakheja, R.; Makis, W.; Skamene, S.; Nahal, A.; Brimo, F.; Azoulay, L.; Assayag, J.; Turcotte, R.; Hickeson, M. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: A retrospective review of 136 patients. AJR Am. J. Roentgenol. 2012, 198, 1409–1416. [Google Scholar] [CrossRef]
- Macpherson, R.E.; Pratap, S.; Tyrrell, H.; Khonsari, M.; Wilson, S.; Gibbons, M.; Whitwell, D.; Giele, H.; Critchley, P.; Cogswell, L.; et al. Retrospective audit of 957 consecutive (18)F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin. Sarcoma Res. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.; Nada, N.; Ghaniem, M.; Elkhamary, S. Assessment of soft tissue tumours of the extremities with diffusion echoplanar MR imaging. Radiol. Med. 2012, 117, 96–101. [Google Scholar] [CrossRef]
- Kim, K.W.; Kuzmiak, C.M.; Kim, Y.J.; Seo, J.Y.; Jung, H.K.; Lee, M.S. Diagnostic Usefulness of Combination of Diffusion-weighted Imaging and T2WI, Including Apparent Diffusion Coefficient in Breast Lesions: Assessment of Histologic Grade. Acad. Radiol. 2018, 25, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Cheng, B.; Ebinger, M.; Hao, Q.; Tourdias, T.; Wu, O.; Kim, J.S.; Breuer, L.; Singer, O.C.; Warach, S.; et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): A multicentre observational study. Lancet Neurol. 2011, 10, 978–986. [Google Scholar] [CrossRef]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.C.; Strauss, D.C.; van Praag, V.V.M.; Levy, A.; Griffin, A.M.; Hayes, A.J.; Stacchiotti, S.; et al. Development and external validation of a dynamic prognostic nomogram for primary extremity soft tissue sarcoma survivors. EClinicalMedicine 2019, 17, 100215. [Google Scholar] [CrossRef]
- Georgiesh, T.; Aggerholm-Pedersen, N.; Schoffski, P.; Zhang, Y.; Napolitano, A.; Bovee, J.; Hjelle, A.; Tang, G.; Spalek, M.; Nannini, M.; et al. Validation of a novel risk score to predict early and late recurrence in solitary fibrous tumour. Br. J. Cancer 2022, 1–6. [Google Scholar] [CrossRef]
- Weigl, H.; Janssen, S.; Vassos, N.; Hohenberger, P.; Simeonova-Chergou, A.; Wenz, F.; Haubenreisser, H.; Jakob, J. Fusion imaging to evaluate the radiographic anatomical relationship between primary tumors and local recurrences in retroperitoneal soft tissue sarcoma. Surg. Oncol. 2020, 34, 109–112. [Google Scholar] [CrossRef]
- Breininger, K.; Albarqouni, S.; Kurzendorfer, T.; Pfister, M.; Kowarschik, M.; Maier, A. Intraoperative stent segmentation in X-ray fluoroscopy for endovascular aortic repair. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1221–1231. [Google Scholar] [CrossRef]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- Peeken, J.C.; Spraker, M.B.; Knebel, C.; Dapper, H.; Pfeiffer, D.; Devecka, M.; Thamer, A.; Shouman, M.A.; Ott, A.; von Eisenhart-Rothe, R.; et al. Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine 2019, 48, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, F.; Dapper, H.; Asadpour, R.; Knebel, C.; Spraker, M.B.; Schwarze, V.; Schaub, S.K.; Mayr, N.A.; Specht, K.; Woodruff, H.C.; et al. Development and External Validation of Deep-Learning-Based Tumor Grading Models in Soft-Tissue Sarcoma Patients Using MR Imaging. Cancers (Basel) 2021, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Snow, H.; Hendry, S.; Mitchell, C.; Slavin, J.; Schlicht, S.; Na, L.; Hofman, M.S.; Gyorki, D.E. Correlation between percutaneous biopsy and final histopathology for retroperitoneal sarcoma: A single-centre study. ANZ J. Surg. 2020, 90, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Wendtland, R.; Dankerl, P.; Bani, M.R.; Fasching, P.A.; Heusinger, K.; Lux, M.P.; Jud, S.M.; Rauh, C.; Bayer, C.M.; Schrauder, M.G.; et al. Evaluation of a Marker Clip System in Sonographically Guided Core Needle Biopsy for Breast Cancer Localization Before and After Neoadjuvant Chemotherapy. Geburtshilfe Frauenheilkd 2017, 77, 169–175. [Google Scholar] [CrossRef] [Green Version]





| Variable | n or Median (Range) | |
|---|---|---|
| Patient | Number of patients | 12 |
| characteristics | Age (years) | 72 (45–79) |
| Sex: Female/Male | 5/7 | |
| Ethnicity: Caucasian/Other | 12/0 | |
| Tumor characteristics | Primary tumor | 10 |
| Recurrent tumor | 2 | |
| Tumor size (cm) | 11.25 (6.2–22) | |
| Histologic subtype | Well-differentiated liposarcoma | 1 |
| Dedifferentiated liposarcoma | 2 | |
| Leiomyosarcoma | 1 | |
| Synovial sarcoma | 1 | |
| Myofibroblastic sarcoma | 2 | |
| MPNST | 1 | |
| Myxofibrosarcoma | 1 | |
| Solitary fibrous tumor | 1 | |
| Undifferentiated Sarcoma, NOS | 2 | |
| Tumor localization | Upper extremity | 3 |
| Lower extremity | 5 | |
| Intraabdominal | 2 | |
| Retroperitoneum | 2 | |
| Grading total tumor | Grade 1 (G1) | 4 |
| (FNCLCC) | Grade 2 (G2) | 6 |
| Grade 3 (G3) | 1 | |
| Grade x (Gx) | 1 |
| Histologic Subtype | Total Tumor | Biopsies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TD | TN | MC | FNCLCC Score | Grading | TD | TN | MC | FNCLCC Score | Grading | |
| Dedifferentiated liposarcoma | 1 | 0 | <10 | 2 | G1 | B1: x | B1: 100% | B1: x | B1: x | B1: x |
| B2: 1 | B2: 0 | B2: 0 | B2: 2 | B2: G1 | ||||||
| B3: 1 | B3: 0 | B3: 0 | B3: 2 | B3: G1 | ||||||
| B4: x | B4: 100% | B4: x | B4: x | B4: x | ||||||
| Synovial sarcoma | 2 | 0 | <10 | 3 | G1 | B1: 2 | B1: 0 | B1: 0 | B1: 3 | B1: G1 |
| B2: 2 | B2: 0 | B2: 0 | B2: 3 | B2: G1 | ||||||
| B3: 2 | B3: 0 | B3: 0 | B3: 3 | B3: G1 | ||||||
| Dedifferentiated liposarcoma | 3 | 0 | <10 | 4 | G2 | B1: 3 | B1: 0 | B1: 0 | B1: 4 | B1: G2 |
| B2: x | B2: 100% | B2: x | B2: x | B2: x | ||||||
| Well-differentiated liposarcoma | 1 | <50% | <10 | 3 | G1 | B1: x | B1: 100% | B1: x | B1: x | B1: x |
| B2: 1 | B2: 0 | B2: 0 | B2: 2 | B2: G1 | ||||||
| B3: 1 | B3: 0 | B3: 0 | B3: 2 | B3: G1 | ||||||
| Myofibroblastic sarcoma | 2 | <50% | 9 | 4 | G2 | B1: 2 | B1: 0 | B1: 0 | B1: 3 | B1: G1 |
| B2: 2 | B2: 0 | B2: 0 | B2: 3 | B2: G1 | ||||||
| B3: 2 | B3: 0 | B3: 0 | B3: 3 | B3: G1 | ||||||
| Leiomyosarcoma | 2 | <50% | 4 | 4 | G2 | B1: 2 | B1: 0 | B1:10 | B1: 4 | B1: G2 |
| B2: 2 | B2: 0 | B2: 2 | B2: 3 | B2: G1 | ||||||
| B3: 2 | B3: 0 | B3: 7 | B3: 3 | B3: G1 | ||||||
| Myxofibrosarcoma | x | x | x | x | Gx | B1: x | B1: x | B1: x | B1: x | B1: Gx |
| B2: x | B2: x | B2: x | B2: x | B2: Gx | ||||||
| Undifferentiated Sarcoma, NOS | 3 | <50% | 1 | 5 | G2 | B1: 3 | B1: 0 | B1: 0 | B1: 4 | B1: G2 |
| B2: 3 | B2: 0 | B2: 0 | B2: 4 | B2: G2 | ||||||
| B3: 3 | B3: <50% | B3: 0 | B3: 5 | B3: G2 | ||||||
| MPNST | 2 | <50% | 0 | 4 | G2 | B1: x | B1: 100% | B1: x | B1: x | B1: x |
| B2: x | B2: 100% | B2: x | B2: x | B2: x | ||||||
| B3: 2 | B3: <50% | B3: 0 | B3: 4 | B3: G2 | ||||||
| Undifferentiated Sarcoma, NOS | 3 | <50% | >20 | 7 | G3 | B1: 3 | B1: 0 | B1:16 | B1: 5 | B1: G2 |
| B2: 3 | B2: 0 | B2: 6 | B2: 4 | B2: G2 | ||||||
| B3: 3 | B3: <50% | B3:15 | B3: 6 | B3: G3 | ||||||
| Myofibroblastic sarcoma | 2 | 0 | 12 | 4 | G2 | B1: 2 | B1: 0 | B1: 1 | B1: 3 | B1: G1 |
| B2: 2 | B2: 0 | B2: 0 | B2: 3 | B2: G1 | ||||||
| B3: 2 | B3: 0 | B3: 2 | B3: 3 | B3: G1 | ||||||
| Solitary fibrous Tumor | 1 | 0 | 2 | 2 | G1 | B1: 1 | B1: 0 | B1: 1 | B1: 2 | B1: G1 |
| B2: 1 | B2: 0 | B2: 1 | B2: 2 | B2: G1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hettler, M.; Kitz, J.; Seif Amir Hosseini, A.; Guhlich, M.; Panahi, B.; Ernst, J.; Conradi, L.-C.; Ghadimi, M.; Ströbel, P.; Jakob, J. Comparing Apparent Diffusion Coefficient and FNCLCC Grading to Improve Pretreatment Grading of Soft Tissue Sarcoma—A Translational Feasibility Study on Fusion Imaging. Cancers 2022, 14, 4331. https://doi.org/10.3390/cancers14174331
Hettler M, Kitz J, Seif Amir Hosseini A, Guhlich M, Panahi B, Ernst J, Conradi L-C, Ghadimi M, Ströbel P, Jakob J. Comparing Apparent Diffusion Coefficient and FNCLCC Grading to Improve Pretreatment Grading of Soft Tissue Sarcoma—A Translational Feasibility Study on Fusion Imaging. Cancers. 2022; 14(17):4331. https://doi.org/10.3390/cancers14174331
Chicago/Turabian StyleHettler, Madelaine, Julia Kitz, Ali Seif Amir Hosseini, Manuel Guhlich, Babak Panahi, Jennifer Ernst, Lena-Christin Conradi, Michael Ghadimi, Philipp Ströbel, and Jens Jakob. 2022. "Comparing Apparent Diffusion Coefficient and FNCLCC Grading to Improve Pretreatment Grading of Soft Tissue Sarcoma—A Translational Feasibility Study on Fusion Imaging" Cancers 14, no. 17: 4331. https://doi.org/10.3390/cancers14174331
APA StyleHettler, M., Kitz, J., Seif Amir Hosseini, A., Guhlich, M., Panahi, B., Ernst, J., Conradi, L. -C., Ghadimi, M., Ströbel, P., & Jakob, J. (2022). Comparing Apparent Diffusion Coefficient and FNCLCC Grading to Improve Pretreatment Grading of Soft Tissue Sarcoma—A Translational Feasibility Study on Fusion Imaging. Cancers, 14(17), 4331. https://doi.org/10.3390/cancers14174331