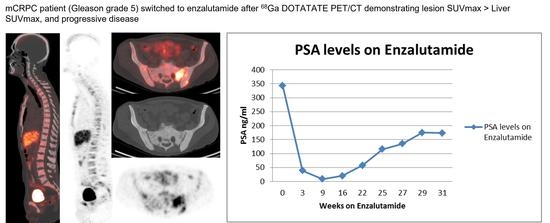
Prognostic Evaluation of Metastatic Castration Resistant Prostate Cancer and Neuroendocrine Prostate Cancer with [68Ga]Ga DOTATATE PET-CT
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.1.1. Subject Recruitment
2.1.2. Image Acquisition
2.1.3. Image Analysis
2.2. Semiquantitative PET and Visual Analysis
2.3. Statistical Analysis
3. Results
Semiquantitative PET and Visual Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Scarpelli, M.; Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; Cascinu, S.; Montironi, R. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: Morphologic and molecular backgrounds and future promises. J. Biol. Regul. Homeost. Agents 2014, 28, 555–563. [Google Scholar] [PubMed]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar] [PubMed]
- Perez, D.; Linares, E.; Almagro, E.; Cantos, B.; Mendez, M.; Maximiano, C.; Franco, F.; Rubio, J.; Palka, M.; Calvo, V.; et al. Neuroendocrine differentiation in carcinoma of the prostate: An institutional review. J. Clin. Oncol. 2014, 15, 32. [Google Scholar] [CrossRef]
- Jimenez, R.E.; Nandy, D.; Qin, R.; Carlson, R.; Tan, W.; Kohli, M. Neuroendocrine differentiation patterns in metastases from advanced prostate cancer. J. Clin. Oncol. 2014, 15, 32. [Google Scholar] [CrossRef]
- Nelson, E.C.; Cambio, A.J.; Yang, J.C.; Ok, J.H.; Lara, P.N., Jr.; Evans, C.P. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic. Dis. 2007, 10, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Borre, M.; Nerstrom, B.; Overgaard, J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin. Cancer Res. 2000, 6, 1882–1890. [Google Scholar]
- Morichetti, D.; Mazzucchelli, R.; Santinelli, A.; Stramazzotti, D.; Lopez-Beltran, A.; Scarpelli, M.; Bono, A.V.; Cheng, L.; Montironi, R. Immunohistochemical expression and localization of somatostatin receptor subtypes in prostate cancer with neuroendocrine differentiation. Int. J. Immunopathol. Pharmacol. 2010, 23, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Montironi, R.; Cheng, L.; Mazzucchelli, R.; Morichetti, D.; Stramazzotti, D.; Santinelli, A.; Moroncini, G.; Galosi, A.B.; Muzzonigro, G.; Comeri, G.; et al. Immunohistochemical detection and localization of somatostatin receptor subtypes in prostate tissue from patients with bladder outlet obstruction. Cell Oncol. 2008, 30, 473–482. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Alonso, O.; Gambini, J.P.; Lago, G.; Gaudiano, J.; Quagliata, A.; Engler, H. In vivo visualization of somatostatin receptor expression with Ga-68-DOTA-TATE PET/CT in advanced metastatic prostate cancer. Clin. Nucl. Med. 2011, 36, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheung, S.K.; Wong, K.N.; Wong, K.K.; Ho, C.L. 68Ga-DOTATOC and 68Ga-PSMA PET/CT Unmasked a Case of Prostate Cancer With Neuroendocrine Differentiation. Clin. Nucl. Med. 2016, 41, 959–960. [Google Scholar] [CrossRef]
- Todorovic-Tirnanic, M.V.; Gajic, M.M.; Obradovic, V.B.; Baum, R.P. Gallium-68 DOTATOC PET/CT in vivo characterization of somatostatin receptor expression in the prostate. Cancer Biother. Radiopharm. 2014, 29, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Gofrit, O.N.; Frank, S.; Meirovitz, A.; Nechushtan, H.; Orevi, M. PET/CT With 68Ga-DOTA-TATE for Diagnosis of Neuroendocrine: Differentiation in Patients With Castrate-Resistant Prostate Cancer. Clin. Nucl. Med. 2017, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Okada, Y.; Minei, S.; Takimoto, Y.; Nemoto, N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur. Urol. 2004, 45, 586–592, discussion 592. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Bogaerts, J.; Ford, R.; Shankar, L.; Therasse, P.; Gwyther, S.; Eisenhauer, E.A. Evaluation of lymph nodes with RECIST 1.1. Eur. J. Cancer 2009, 45, 261–267. [Google Scholar] [CrossRef]
- Menon, B.K.; Kalshetty, A.; Bhattacharjee, A.; Basu, S. Standardized uptake values and ratios on 68Ga-DOTATATE PET-computed tomography for normal organs and malignant lesions and their correlation with Krenning score in patients with metastatic neuroendocrine tumors. Nucl. Med. Commun. 2020, 41, 1095–1099. [Google Scholar] [CrossRef]
- Komiya, A.; Yasuda, K.; Watanabe, A.; Fujiuchi, Y.; Tsuzuki, T.; Fuse, H. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Mol. Clin. Oncol. 2013, 1, 257–262. [Google Scholar] [CrossRef] [Green Version]
- di Sant’Agnese, P.A. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer 1992, 70, 254–268. [Google Scholar] [CrossRef]
- di Sant’Agnese, P.A. Neuroendocrine differentiation in prostatic carcinoma: An update on recent developments. Ann. Oncol. 2001, 12 (Suppl. 2), S135–S140. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Rindi, G.; Papotti, M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: A comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows. Arch. 2006, 449, 499–506. [Google Scholar] [CrossRef]
- Beltran, H.; Tomlins, S.; Aparicio, A.; Arora, V.; Rickman, D.; Ayala, G.; Huang, J.; True, L.; Gleave, M.E.; Soule, H.; et al. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 2846–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: A mechanism of radioresistance and treatment failure. Front. Oncol. 2015, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.U.; Costa, D.N.; Jia, L.; Oz, O.K.; de Blanche, L. 68Ga-DOTATATE PET/CT Uptake in Prostate With an Incidental Finding of Prostatic Acinar Adenocarcinoma and Metastatic Neuroendocrine Cancer to the Liver. Clin. Nucl. Med. 2021, 46, e428–e430. [Google Scholar] [CrossRef] [PubMed]
- Acar, E.; Kaya, G.C. 18F-FDG, 68Ga-DOTATATE and 68Ga-PSMA Positive Metastatic Large Cell Neuroendocrine Prostate Tumor. Clin. Nucl. Med. 2019, 44, 53–54. [Google Scholar] [CrossRef]
- Wang, J. 68Ga-DOTATATE in Benign Prostate Hyperplasia. Clin. Nucl. Med. 2019, 44, 249–250. [Google Scholar] [CrossRef]
- Schmidt, M.Q.; Trenbeath, Z.; Chin, B.B. Neuroendocrine prostate cancer or prostatitis? An unusual false positive on gallium-68 DOTA-Tyr3-octreotate positron emission tomography/computed tomography in a patient with known metastatic neuroendocrine tumor. World J. Nucl. Med. 2019, 18, 304–306. [Google Scholar] [CrossRef]
- Assadi, M.; Pirayesh, E.; Rekabpour, S.J.; Zohrabi, F.; Jafari, E.; Nabipour, I.; Esmaili, A.; Amini, A.; Ahmadzadehfar, H. 177Lu-PSMA and 177Lu-DOTATATE Therapy in a Patient With Metastatic Castration-Resistant Prostate Cancer and Neuroendocrine Differentiation. Clin. Nucl. Med. 2019, 44, 978–980. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, J.; Baum, R.P.; Yang, Z. Excellent Response to 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy in a Patient With Progressive Metastatic Castration-Resistant Prostate Cancer With Neuroendocrine Differentiation After 177Lu-PSMA Therapy. Clin. Nucl. Med. 2019, 44, 876–878. [Google Scholar] [CrossRef]
- Dos Santos, G.; Garcia Fontes, M.; Engler, H.; Alonso, O. Intraindividual comparison of (68)Ga-DOTATATE PET/CT vs. (11)C-Choline PET/CT in patients with prostate cancer in biochemical relapse: In vivo evaluation of the expression of somatostatin receptors. Rev. Esp. Med. Nucl. Imagen Mol. (Engl. Ed.) 2019, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]




| Gleason Grade | PSA at Time of PET/CT (ng/mL) | Index Lesions with [68Ga]Ga DOTATATE Uptake | SUVmax Hottest Lesion | Systemic Treatments Prior to CRPC Diagnosis | Additional Systemic Treatments after CRPC Diagnosis |
|---|---|---|---|---|---|
| 4 | 39.23 | 7 | 2.1 | ADT alone | Enzalutamide |
| 3 | 11.18 | 7 | 3.1 | ADT alone | Enzalutamide |
| 2 | 7.09 | 3 | 3.3 | ADT alone | Enzalutamide |
| N/A * | 48.08 | 6 | 6.2 | ADT alone | Docetaxel |
| 5 | 4.4 | 2 | 6.6 | ADT alone | Enzalutamide |
| 5 | 44.54 | 8 | 7.3 | ADT alone | Docetaxel |
| 5 | 38.99 | 9 | 8.3 | ADT+ Docetaxel + anti-androgen therapy | Abiraterone |
| 5 | 1033.87 | 7 | 8.6 | ADT+ Abiraterone | cabazitaxol + carboplatin |
| 5 | 28.76 | 9 | 8.9 | ADT+ Docetaxel + anti-androgen therapy | Abiraterone |
| 5 | 31.37 | 6 | 10.7 | ADT alone | Docetaxel |
| 5 | 115.45 | 5 | 11 | ADT+ Docetaxel + anti-androgen therapy | Cabazitaxol |
| 5 † | 26.51 | 8 | 20.1 | ADT + platinium/etoposide | Enzalutamide |
| 4 | 128 | 6 | 20.2 | ADT+ Abiraterone | Docetaxel |
| 5 | 61.86 | 9 | 23.1 | ADT alone | Enzalutamide |
| 2 | 88.05 | 13 | 27 | ADT+ Abiraterone | Docetaxel |
| N/A ‡ | <0.01 | 8 | 28.5 | platinium+ etoposide | Cisplatin + etoposide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilen, M.A.; Akintayo, A.; Liu, Y.; Abiodun-Ojo, O.; Kucuk, O.; Carthon, B.C.; Schuster, D.M.; Parent, E.E. Prognostic Evaluation of Metastatic Castration Resistant Prostate Cancer and Neuroendocrine Prostate Cancer with [68Ga]Ga DOTATATE PET-CT. Cancers 2022, 14, 6039. https://doi.org/10.3390/cancers14246039
Bilen MA, Akintayo A, Liu Y, Abiodun-Ojo O, Kucuk O, Carthon BC, Schuster DM, Parent EE. Prognostic Evaluation of Metastatic Castration Resistant Prostate Cancer and Neuroendocrine Prostate Cancer with [68Ga]Ga DOTATATE PET-CT. Cancers. 2022; 14(24):6039. https://doi.org/10.3390/cancers14246039
Chicago/Turabian StyleBilen, Mehmet Asim, Akinyemi Akintayo, Yuan Liu, Olayinka Abiodun-Ojo, Omer Kucuk, Bradley C. Carthon, David M. Schuster, and Ephraim E. Parent. 2022. "Prognostic Evaluation of Metastatic Castration Resistant Prostate Cancer and Neuroendocrine Prostate Cancer with [68Ga]Ga DOTATATE PET-CT" Cancers 14, no. 24: 6039. https://doi.org/10.3390/cancers14246039
APA StyleBilen, M. A., Akintayo, A., Liu, Y., Abiodun-Ojo, O., Kucuk, O., Carthon, B. C., Schuster, D. M., & Parent, E. E. (2022). Prognostic Evaluation of Metastatic Castration Resistant Prostate Cancer and Neuroendocrine Prostate Cancer with [68Ga]Ga DOTATATE PET-CT. Cancers, 14(24), 6039. https://doi.org/10.3390/cancers14246039