Longitudinal Analysis of 1α,25-dihidroxyvitamin D3 and Homocysteine Changes in Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
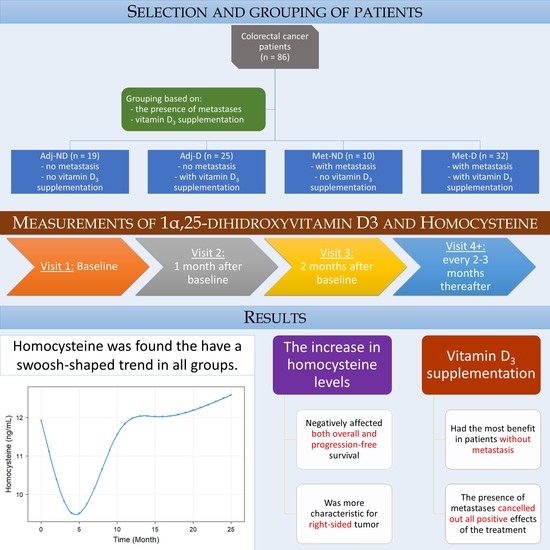
2.1. Study Design and Patient Selection
2.2. Clinicopathological and Laboratory Data Measurements
2.3. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics and Measurements
3.2. Analysis of Longitudinal Data
3.3. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Marcinkowska, E.; Wallace, G.R.; Brown, G. The Use of 1alpha,25-Dihydroxyvitamin D(3) as an Anticancer Agent. Int. J. Mol. Sci. 2016, 17, 729. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Sato, K.; Hollis, B.W.; Zhang, S.; Niedzwiecki, D.; Ou, F.S.; Chang, I.W.; O’Neil, B.H.; Innocenti, F.; Lenz, H.J.; et al. Plasma 25-Hydroxyvitamin D Levels and Survival in Patients with Advanced or Metastatic Colorectal Cancer: Findings from CALGB/SWOG 80405 (Alliance). Clin. Cancer Res. 2019, 25, 7497–7505. [Google Scholar] [CrossRef] [Green Version]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Dou, R.; Ng, K.; Giovannucci, E.L.; Manson, J.E.; Qian, Z.R.; Ogino, S. Vitamin D and colorectal cancer: Molecular, epidemiological and clinical evidence. Br. J. Nutr. 2016, 115, 1643–1660. [Google Scholar] [CrossRef] [Green Version]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment. Pharmacol. Ther. 2009, 30, 113–125. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef]
- Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Munoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. Mol. Biol. 2019, 185, 1–6. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Raoul, P.; Cintoni, M.; Gasbarrini, A. Vitamin D and colorectal cancer: Chemopreventive perspectives through the gut microbiota and the immune system. Biofactors 2021, in press. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Koole, J.L.; Bours, M.J.L.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; van Duijnhoven, F.J.B.; van den Ouweland, J.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Weijenberg, M.P. Higher Serum Vitamin D Concentrations Are Longitudinally Associated with Better Global Quality of Life and Less Fatigue in Colorectal Cancer Survivors up to 2 Years after Treatment. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan-Shaw, P.G.; Zgaga, L.; Ooi, L.Y.; Theodoratou, E.; Timofeeva, M.; Svinti, V.; Walker, M.; O’Sullivan, F.; Ewing, A.; Johnston, S.; et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut 2020, 69, 103–111. [Google Scholar] [CrossRef]
- Mezawa, H.; Sugiura, T.; Watanabe, M.; Norizoe, C.; Takahashi, D.; Shimojima, A.; Tamez, S.; Tsutsumi, Y.; Yanaga, K.; Urashima, M. Serum vitamin D levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer 2010, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, A.S. Homocysteine and cardiovascular disease: A review of the evidence. Diab. Vasc. Dis. Res. 2007, 4, 143–150. [Google Scholar] [CrossRef]
- Amer, M.; Qayyum, R. The relationship between 25-hydroxyvitamin D and homocysteine in asymptomatic adults. J. Clin. Endocrinol. Metab. 2014, 99, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Keshteli, A.H.; Baracos, V.E.; Madsen, K.L. Hyperhomocysteinemia as a potential contributor of colorectal cancer development in inflammatory bowel diseases: A review. World J. Gastroenterol. 2015, 21, 1081–1090. [Google Scholar] [CrossRef]
- Chiang, F.F.; Wang, H.M.; Lan, Y.C.; Yang, M.H.; Huang, S.C.; Huang, Y.C. High homocysteine is associated with increased risk of colorectal cancer independently of oxidative stress and antioxidant capacities. Clin. Nutr. 2014, 33, 1054–1060. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, J.H.; Park, S.K.; Son, H.J.; Kim, J.J.; Kim, Y.H. Hyperhomocysteinemia is a risk factor for colorectal adenoma in women. J. Clin. Biochem. Nutr. 2012, 51, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Xue, L.; Zhu, G.; Chen, Y. Serum Homocysteine, VEGF and TGF-β1 dynamic change in colorectal cancer patients prior and post-operation. Pteridines 2019, 30, 121–125. [Google Scholar] [CrossRef]
- Schwandt, A.; Denkinger, M.; Fasching, P.; Pfeifer, M.; Wagner, C.; Weiland, J.; Zeyfang, A.; Holl, R.W. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J. Diabetes Complicat. 2017, 31, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Jessup, J.; Goldberg, R.; Asare, E.; Benson, A.; Brierley, J.; Chang, G.; Chen, V.; Compton, C.; De Nardi, P.; Goodman, K.; et al. Colon and Rectum. In AJCC Cancer Staging Manual, 8th ed.; Amin, M., Edge, S., Greene, F., Byrd, D., Brookland, R., Washington, M., Gershenwald, J., Compton, C., Hess, K., Sullivan, D., et al., Eds.; Springer International Publishing: Chicago, IL, USA, 2018; pp. 251–274. [Google Scholar]
- Shen, H.; Yang, J.; Huang, Q.; Jiang, M.J.; Tan, Y.N.; Fu, J.F.; Zhu, L.Z.; Fang, X.F.; Yuan, Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J. Gastroenterol. 2015, 21, 6470–6478. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Park, Y.C.; Kim, J.; Seo, M.S.; Hong, S.W.; Cho, E.S.; Kim, J.K. Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology 2017, 22, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Glueck, C.J.; Jetty, V.; Rothschild, M.; Duhon, G.; Shah, P.; Prince, M.; Lee, K.; Goldenberg, M.; Kumar, A.; Goldenberg, N.; et al. Associations between Serum 25-hydroxyvitamin D and Lipids, Lipoprotein Cholesterols, and Homocysteine. N. Am. J. Med. Sci. 2016, 8, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- van Guldener, C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrol. Dial. Transplant. 2006, 21, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Carru, C.; Deiana, L.; Sotgia, S.; Usai, M.F.; Zinellu, A. Relationships between white blood cell count and levels of serum homocysteine and cysteine in healthy subjects. Haematologica 2005, 90, 136–137. [Google Scholar]
- Gopinath, B.; Rochtchina, E.; Flood, V.; Mitchell, P. Association of elevated homocysteine level and vitamin B12 deficiency with anemia in older adults. Arch. Intern. Med. 2009, 169, 901–902. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.A. Vitamin D: A hormone for all seasons—How much is enough? Clin. Biochem. Rev. 2005, 26, 21–32. [Google Scholar]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianferotti, L.; Bertoldo, F.; Bischoff-Ferrari, H.A.; Bruyere, O.; Cooper, C.; Cutolo, M.; Kanis, J.A.; Kaufman, J.M.; Reginster, J.Y.; Rizzoli, R.; et al. Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: Research for evidence and a scientific statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Endocrine 2017, 56, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Savoie, M.B.; Paciorek, A.; Zhang, L.; Van Blarigan, E.L.; Sommovilla, N.; Abrams, D.; Atreya, C.E.; Bergsland, E.K.; Chern, H.; Kelley, R.K.; et al. Vitamin D Levels in Patients with Colorectal Cancer Before and After Treatment Initiation. J. Gastrointest. Cancer 2019, 50, 769–779. [Google Scholar] [CrossRef]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of High-Dose vs. Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, K.; Zhao, T.; Sun, C.; Patel, A.S.; Liu, H.; Chen, X.; Qu, G.; Sun, Y. The association between hypertension and colorectal cancer: A meta-analysis of observational studies. Eur. J. Cancer Prev. 2021, 30, 84–96. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, N.; Prieto, I.; Del Puerto-Nevado, L.; Portal-Nunez, S.; Ardura, J.A.; Corton, M.; Fernandez-Fernandez, B.; Aguilera, O.; Gomez-Guerrero, C.; Mas, S.; et al. 2017 update on the relationship between diabetes and colorectal cancer: Epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget 2017, 8, 18456–18485. [Google Scholar] [CrossRef] [Green Version]
- Alkhatatbeh, M.J.; Amara, N.A.; Abdul-Razzak, K.K. Association of 25-hydroxyvitamin D with HDL-cholesterol and other cardiovascular risk biomarkers in subjects with non-cardiac chest pain. Lipids Health Dis. 2019, 18, 27. [Google Scholar] [CrossRef] [Green Version]
- Karhapaa, P.; Pihlajamaki, J.; Porsti, I.; Kastarinen, M.; Mustonen, J.; Niemela, O.; Kuusisto, J. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J. Intern. Med. 2010, 268, 604–610. [Google Scholar] [CrossRef]
- Vladimirov, S.; Zeljkovic, A.; Gojkovic, T.; Miljkovic, M.; Stefanovic, A.; Zeljkovic, D.; Trifunovic, B.; Spasojevic-Kalimanovska, V. Associations of cholesterol and vitamin D metabolites with the risk for development of high grade colorectal cancer. J. Med. Biochem. 2020, 39, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, K.; Fujimoto, T.; Fujigaki, Y.; Hishida, A. Vitamin D deficiency is implicated in reduced serum albumin concentrations in patients with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, R.; Hoenderop, J.G.; Stavenuiter, A.W.; Ferrantelli, E.; Baltissen, M.P.; Dijkman, H.B.; Florquin, S.; Rops, A.L.; Wetzels, J.F.; Berden, J.H.; et al. 1,25-Vitamin D3 Deficiency Induces Albuminuria. Am. J. Pathol. 2016, 186, 794–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.C.; You, J.F.; Yeh, C.Y.; Chen, J.S.; Tang, R.; Wang, J.Y.; Chin, C.C. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int. J. Colorectal Dis. 2011, 26, 473–481. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, C.; Wang, X.; Fernandez-Escobar, A.; Wu, Q.; Xu, K.; Mao, J.; Jin, M.; Wang, K. Plasma Levels of Homocysteine and the Occurrence and Progression of Rectal Cancer. Med. Sci. Monit. 2018, 24, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Momin, M.; Jia, J.; Fan, F.; Li, J.; Dou, J.; Chen, D.; Huo, Y.; Zhang, Y. Relationship between plasma homocysteine level and lipid profiles in a community-based Chinese population. Lipids Health Dis. 2017, 16, 54. [Google Scholar] [CrossRef] [Green Version]
- Sakuta, H.; Suzuki, T.; Yasuda, H.; Ito, T. White blood cell count is associated with plasma total homocysteine in Japanese men. Scand. J. Clin. Lab. Investig. 2005, 65, 447–452. [Google Scholar] [CrossRef]
- Undas, A.; Stepien, E.; Plicner, D.; Zielinski, L.; Tracz, W. Elevated total homocysteine is associated with increased platelet activation at the site of microvascular injury: Effects of folic acid administration. J. Thromb. Haemost. 2007, 5, 1070–1072. [Google Scholar] [CrossRef]
- Mohan, I.V.; Jagroop, I.A.; Mikhailidis, D.P.; Stansby, G.P. Homocysteine activates platelets in vitro. Clin. Appl. Thromb. Hemost. 2008, 14, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Wang, T.; Ye, C.; Xu, G.; Xie, L. Relationship between lactate dehydrogenase and albuminuria in Chinese hypertensive patients. J. Clin. Hypertens. 2021, 23, 128–136. [Google Scholar] [CrossRef]
- Huang, J.W.; Yen, C.J.; Pai, M.F.; Wu, K.D.; Tsai, T.J.; Hsieh, B.S. Association between serum aspartate transaminase and homocysteine levels in hemodialysis patients. Am. J. Kidney Dis. 2002, 40, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.E.; Eggers, P.W.; Hostetter, T.H.; Briggs, J.P. Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int. 2004, 66, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Lu, Y.; Deng, Y.; Xu, J.; Zhang, X. Homocysteine level is positively and independently associated with serum creatinine and urea nitrogen levels in old male patients with hypertension. Sci. Rep. 2020, 10, 18050. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Ren, J.; Huang, J.; Li, D. Association of homocysteine with type 2 diabetes: A meta-analysis implementing Mendelian randomization approach. BMC Genom. 2013, 14, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, D.E.; Hariri, E.; Salameh, P.; Merhi, M.; Sabbah, N.; Helou, M.; Mouzaya, F.; Nemer, R.; Al-Sarraj, Y.; El-Shanti, H.; et al. Type II diabetes mellitus and hyperhomocysteinemia: A complex interaction. Diabetol. Metab. Syndr. 2017, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Hubner, U.; Geisel, J.; Kirsch, S.H.; Kruse, V.; Bodis, M.; Klein, C.; Herrmann, W.; Obeid, R. Effect of 1 year B and D vitamin supplementation on LINE-1 repetitive element methylation in older subjects. Clin. Chem. Lab. Med. 2013, 51, 649–655. [Google Scholar] [CrossRef]
- Hollender, A.; Bjoro, T.; Otto Karlsen, K.; Kvaloy, S.O.; Nome, O.; Holte, H. Vitamin D deficiency in patients operated on for gastric lymphoma. Scand. J. Gastroenterol. 2006, 41, 673–681. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D deficiency increases severity of paclitaxel-induced peripheral neuropathy. Breast Cancer Res. Treat 2020, 180, 707–714. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, R.; Won, H.; Kim, S.; Choi, H.J.; Ryu, J.M.; Lee, S.K.; Yu, J.; Kim, S.W.; Lee, J.E.; et al. Serum Vitamin Levels and Their Relationships with Other Biomarkers in Korean Breast Cancer Patients. Nutrients 2020, 12, 2831. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Adj-ND (n = 19) | Adj-D (n = 25) | Met-ND (n = 10) | Met-D (n = 32) |
|---|---|---|---|---|
| Age (years) | 64.87 ± 10.37 | 62.58 ± 8.62 | 65.59 ± 7.16 | 59.49 ± 11.96 |
| Sex (Male:Female) | 12:7 (63.2%:36.8%) | 15:10 (60.0%:40.0%) | 7:3 (70.0%:30.0%) | 20:12 (62.5%:37.5%) |
| Location of the tumor | ||||
| - Right-sided | 8 (42.1%) | 12 (48.0%) | 1 (10.0%) | 8 (25.0%) |
| - Left-sided 1 | 11 (57.9%) | 13 (52.0%) | 9 (90.0%) | 24 (75.0%) |
| Staging (AJCC [22]) 2 | ||||
| - Stage I | 0 (0.0%) | 2 (8.0%) | 0 (0.0%) | 0 (0.0%) |
| - Stage II | 8 (42.1%) | 10 (40.0%) | 0 (0.0%) | 0 (0.0%) |
| - Stage III | 11 (57.9%) | 13 (52.0%) | 0 (0.0%) | 0 (0.0%) |
| - Stage IV | 0 (0.0%) | 0 (0.0%) | 10 (100.0%) | 32 (100.0%) |
| Distant metastasis developed later with the course of the disease | 3 (15.8%) | 4 (16.0%) | – | – |
| Chemotherapy | ||||
| - Adjuvant | 17 (89.5%) | 22 (88.0%) | 0 (0.0%) | 0 (0.0%) |
| - Metastatic | ||||
| o First line | 2 (10.5%) | 3 (12.0%) | 4 (40.0%) | 12 (37.5%) |
| o Second line | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | 10 (31.25%) |
| o Third line or above | 0 (0.0%) | 0 (0.0%) | 5 (50.0%) | 10 (31.25%) |
| Medical history | ||||
| - Type 2 diabetes mellitus | 6 (31.6%) | 6 (24.0%) | 2 (20.0%) | 5 (15.6%) |
| - Hypertension | 12 (63.2%) | 16 (64.0%) | 9 (90.0%) | 18 (56.3%) |
| - Cardiovascular diseases | 3 (15.8%) | 6 (24.0%) | 3 (30.0%) | 4 (12.5%) |
| - Cardiovascular event(s) | 2 (10.5%) | 3 (12.0%) | 0 (0.0%) | 4 (12.5%) |
| - Thyroid diseases 3 | 2 (10.5%) | 3 (12.0%) | 3 (30.0%) | 4 (12.5%) |
| Disease progression 4 | 3 (15.8%) | 4 (16.0%) | 9 (90.0%) | 28 (87.5%) |
| CRC-related death 5 | 1 (5.3%) | 1 (4.0%) | 9 (90.0%) | 25 (78.1%) |
| Parameter | Adj-ND (n = 19) | Adj-D (n = 25) | Met-ND (n = 10) | Met-D (n = 32) |
|---|---|---|---|---|
| White blood cell count (109/L) | 7.51 ± 3.17 | 6.80 ± 1.70 | 8.15 ± 2.45 | 7.73 ± 3.22 |
| Red blood cell count (1012/L) | 4.52 ± 0.48 | 4.55 ± 0.47 | 4.35 ± 0.37 | 4.61 ± 0.52 |
| Hemoglobin (g/L) | 124.95 ± 18.32 | 126.04 ± 15.27 | 127.00 ± 19.09 | 130.38 ± 18.17 |
| Hematocrit (L/L) | 0.38 ± 0.05 | 0.39 ± 0.04 | 0.38 ± 0.04 | 0.39 ± 0.05 |
| Platelet count (109/L) | 231.26 ± 60.30 | 269.96 ± 86.74 | 332.70 ± 130.46 | 294.25 ± 132.94 |
| Aspartate transaminase (U/L) | 22.11 ± 4.70 | 24.58 ± 8.60 | 32.90 ± 19.54 | 50.00 ± 73.38 |
| Alanine transaminase (U/L) | 21.00 ± 9.46 | 23.12 ± 13.26 | 27.30 ± 20.47 | 38.50 ± 32.42 |
| Gamma-glutamyl transferase (U/L) | 34.50 ± 17.84 | 30.42 ± 12.83 | 155.50 ± 261.67 | 137.53 ± 163.50 |
| Lactate dehydrogenase (U/L) | 182.68 ± 28.79 * | 178.50 ± 38.61 * | 357.00 ± 291.13 * | 587.44 ± 1183.24 * |
| Creatinine (µmol/L) | 79.00 ± 18.95 | 69.04 ± 14.06 | 68.80 ± 21.66 | 64.69 ± 12.67 |
| Estimated glomerular filtration rate | 82.74 ± 18.38 | 90.71 ± 14.03 | 89.52 ± 21.13 | 97.48 ± 13.10 |
| Total cholesterol (mmol/L) | 5.16 ± 1.24 | 5.50 ± 1.28 | 6.35 ± 2.24 | 5.59 ± 1.45 |
| High-density lipoprotein (mmol/L) | 1.54 ±0.51 | 1.45 ± 0.33 | 1.37 ± 0.34 | 1.37 ± 0.44 |
| Low-density lipoprotein (mmol/L) | 3.05 ± 0.80 | 3.39 ± 0.93 | 3.92 ± 1.43 | 3.54 ± 1.03 |
| Triglyceride (mmol/L) | 1.39 ± 0.99 | 1.64 ± 0.82 | 1.63 ± 0.89 | 1.58 ± 0.52 |
| High-sensitivity C-reactive protein (mg/L) | 3.93 ± 3.70 * | 3.57 ± 3.50 * | 26.90 ± 35.16 * | 21.80 ± 43.92 * |
| Total protein (g/L) | 75.58 ± 3.87 | 76.40 ± 4.55 | 73.98 ± 3.98 | 72.98 ± 5.35 |
| Albumin (g/L) | 44.24 ± 2.46 | 44.30 ± 3.13 | 42.53 ± 4.68 | 42.94 ± 3.48 |
| Carcinoembryonic antigen (ng/mL) | 2.33 ± 1.27 * | 2.01 ± 1.48 * | 117.21 ± 162.72 * | 367.81 ± 1496.30 * |
| Carbohydrate antigen 19-9 (U/mL) | 6.53 ± 11.39 * | 7.46 ± 12.98 * | 451.60 ± 813.46 * | 925.56 ± 3656.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mühl, D.; Herold, M.; Herold, Z.; Hornyák, L.; Szasz, A.M.; Dank, M. Longitudinal Analysis of 1α,25-dihidroxyvitamin D3 and Homocysteine Changes in Colorectal Cancer. Cancers 2022, 14, 658. https://doi.org/10.3390/cancers14030658
Mühl D, Herold M, Herold Z, Hornyák L, Szasz AM, Dank M. Longitudinal Analysis of 1α,25-dihidroxyvitamin D3 and Homocysteine Changes in Colorectal Cancer. Cancers. 2022; 14(3):658. https://doi.org/10.3390/cancers14030658
Chicago/Turabian StyleMühl, Dorottya, Magdolna Herold, Zoltan Herold, Lilla Hornyák, Attila Marcell Szasz, and Magdolna Dank. 2022. "Longitudinal Analysis of 1α,25-dihidroxyvitamin D3 and Homocysteine Changes in Colorectal Cancer" Cancers 14, no. 3: 658. https://doi.org/10.3390/cancers14030658
APA StyleMühl, D., Herold, M., Herold, Z., Hornyák, L., Szasz, A. M., & Dank, M. (2022). Longitudinal Analysis of 1α,25-dihidroxyvitamin D3 and Homocysteine Changes in Colorectal Cancer. Cancers, 14(3), 658. https://doi.org/10.3390/cancers14030658