Nutritional Interventions in Pancreatic Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- P(opulation): pancreatic cancer patients;
- I(ntervention): nutrition intervention (PN, EN, DS, mixed or special forms);
- C(omparison): no nutrition intervention or placebo or alternative treatment;
- O(utcome): malnutrition or cachexia or weight loss.
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Quality Criteria
2.5. Data Extraction and Analysis
3. Results
3.1. Overview
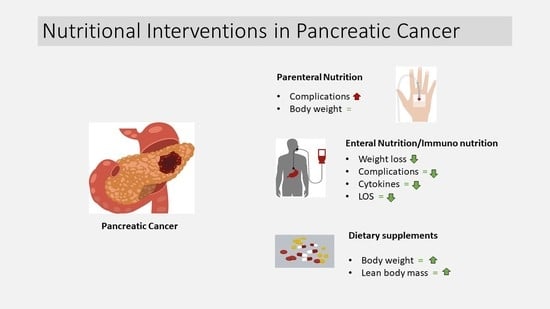
3.2. Parenteral Nutrition
3.3. Enteral Nutrition
3.4. Dietary Supplements
3.5. Mixed and Special Forms of Nutritional Interventions
3.6. Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seufferlein, T.; Porzner, M.; Becker, T.; Budach, V.; Ceyhan, G.; Esposito, I.; Fietkau, R.; Follmann, M.; Friess, H.; Galle, P.; et al. S3-Leitlinie zum exokrinen Pankreaskarzinom. Z. Gastroenterol. 2013, 51, 1395–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, K.C.; Klimstra, D.S.; Brennan, M.F. Long-Term Survival after Curative resection for Pancreatic Ductal Adenocarcinoma: Clinicopathologic Analysis of 5-Year Survivors. Ann. Surg. 1996, 223, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Doi, R.; Imaizumi, T.; Funakoshi, A.; Wakasugi, H.; Sunamura, M.; Ogata, Y.; Hishinuma, S.; Asano, T.; Aikou, T.; et al. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery 2004, 136, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, C.A.; Friess, H.; Büchler, M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Baracos, V.E. Cachexia in pancreatic cancer: New treatment options and measures of success. HPB (Oxford) 2010, 12, 323–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced panreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Davidson, W.; Ash, S.; Capra, S.; Bauer, J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin. Nutr. 2004, 23, 239–247. [Google Scholar] [CrossRef]
- Richter, E.; Denecke, A.; Klapdor, S.; Klapdor, R. Parenteral Nutrition Support for Patients with Pancreatic Cancer—Improvement of the Nutritional Status and the Therapeutic Outcome. Anticancer Res. 2012, 32, 2111–2118. [Google Scholar]
- Bosaeus, I.; Daneryd, P.; Svanberg, E.; Lundholm, K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int. J. Cancer 2001, 93, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.C.; Barber, M.D.; Falconer, J.S.; McMillan, D.C.; Ross, J.A.; Preston, T. Pancreatic cancer as a model: Inflammatory mediators, acute-phase response, and cancer cachexia. World J. Surg. 1999, 23, 584–588. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi-Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome--when all you can eat is yourself. Nat. Clin. Pract. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk, O.; Steinacker, J.M.; Nitsche, U.; Otto, S.; Bachmann, J.; Schubert, E.C.; Friess, H.; Martignoni, M.E. IL-4 mRNA Is Downregulated in the Liver of Pancreatic Cancer Patients Suffering from Cachexia. Nutr. Cancer 2017, 69, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.-C.; Handschel, J.; Holtmann, H.; Krüskemper, G. Oral cancer malnutrition impacts weight and quality of life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Miyake, M.; Hama, N.; Miyamoto, A.; Ikeda, M.; et al. Prevalence of Malnutrition among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), 778–785. [Google Scholar] [CrossRef]
- Latenstein, A.E.J.; Dijksterhuis, W.P.M.; Mackay, T.M.; Beijer, S.; van Eijck, C.H.J.; de Hingh, I.H.J.T.; Molenaar, I.Q.; van Oijen, M.G.H.; van Santvoort, H.C.; de van der Schueren, M.A.E.; et al. Cachexia, dietetic consultation, and survival in patients with pancreatic and periampullary cancer: A multicenter cohort study. Cancer Med. 2020, 9, 9385–9395. [Google Scholar] [CrossRef]
- Vashi, P.; Popiel, B.; Lammersfeld, C.; Gupta, D. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas 2015, 44, 750–755. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Arends, J.; Bertz, H.; Bischoff, S.; Fietkau, R.; Herrmann, H.; Holm, E.; Horneber, M.; Hütterer, E.; Körber, J.; Schmid, I. S3-Leitline der Deutschen Gesellschaft für Ernährungsmedizin e. V. (DGEM) in Kooperation mit der Deutschen Gesellschaft für Hämatologie und Onkologie e. V. (DGHO), der Arbeitsgemeinschaft “Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin" der Deutschen Krebsgesellschaft (ASORS) und der Österreichischen Arbeitsgemeinschaft für klinische Ernährung (AKE). Aktuel Ernahr. 2015, 40, e1–e74. [Google Scholar] [CrossRef] [Green Version]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN Guidelines on Parenteral Nutrition: Non-surgical oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, L.; Volkert, D.; Schütz, T.; Ockenga, J.; Pirlich, M.; Druml, W.; Schindler, K.; Ballmer, P.; Bischoff, S.; Weimann, A.; et al. Leitlinie der Deutschen Gesellschaft für Ernährungsmedizin (DGEM). Aktuel Ernahr. 2013, 38, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.-C.; Roberts, G.P.; Meek, C.L.; et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019, 29, 707–718.e8. [Google Scholar] [CrossRef] [Green Version]
- Aotani, N.; Yasui-Yamada, S.; Kagiya, N.; Takimoto, M.; Oiwa, Y.; Matsubara, A.; Matsuura, S.; Tanimura, M.; Tani-Suzuki, Y.; Kashihara, H.; et al. Malnutrition by European Society for Clinical Nutrition and Metabolism criteria predicts prognosis in patients with gastrointestinal and hepatobiliary-pancreatic cancer. Clin. Nutr. ESPEN 2021, 42, 265–271. [Google Scholar] [CrossRef]
- Arends, J. Ernährung von Tumorpatienten. Aktuel Ernahr. 2012, 37, 91–106. [Google Scholar] [CrossRef]
- Kasper, H. Ernährungsmedizin und Diätetik, 12., überarb. Aufl.; Elsevier, Urban & Fischer: München, Germany, 2014; ISBN 9783437420139. [Google Scholar]
- Prado, C.M.; Anker, S.D.; Coats, A.J.S.; Laviano, A.; von Haehling, S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J. Cachexia Sarcopenia Muscle 2020, 12, 3. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic Review of Peri-Operative Nutritional Supplementation in Patients undergoing Pancreaticoduodenectomy. J. Pancreas 2006, 7, 5–13. [Google Scholar]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran, C.H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy. Medicine 2018, 97, e0472. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Yu, J.; Xiao, J.; Cao, B.W. The consumption of omega-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: A systematic evaluation. Nutr. Cancer 2015, 67, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Clarke, L.; Goldberg, A.; Bishop, K.S. Pancreatic Cancer Cachexia: The Role of Nutritional Interventions. Healthcare 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Adiamah, A.; Skořepa, P.; Weimann, A.; Lobo, D.N. The Impact of Preoperative Immune Modulating Nutrition on Outcomes in Patients Undergoing Surgery for Gastrointestinal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 247–256. [Google Scholar] [CrossRef]
- Yang, F.-A.; Chen, Y.-C.; Tiong, C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2798. [Google Scholar] [CrossRef]
- Kim, A.J.; Hong, D.S.; George, G.C. Diet-related interventions for cancer-associated cachexia. J. Cancer Res. Clin. Oncol. 2021, 147, 1443–1450. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Teleni, L.; McCarthy, A.L.; Isenring, E.A. Cancer cachexia: An overview of diagnostic criteria and therapeutic approaches for the accredited practicing dietitian. J. Hum. Nutr. Diet. 2020, 34, 243–254. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 9781119536628. [Google Scholar]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Takahashi, H.; Asukai, K.; Tomokuni, A.; Wada, H.; Marukawa, S.; Yamasaki, T.; Yanagimoto, Y.; Takahashi, Y.; Sugimura, K.; et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin. Nutr. ESPEN 2019, 33, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ashida, R.; Okamura, Y.; Wakabayashi-Nakao, K.; Mizuno, T.; Aoki, S.; Uesaka, K. The Impact of Preoperative Enteral Nutrition Enriched with Eicosapentaenoic Acid on Postoperative Hypercytokinemia after Pancreatoduodenectomy: The Results of a Double-Blinded Randomized Controlled Trial. Dig. Surg. 2019, 36, 348–356. [Google Scholar] [CrossRef]
- Bauer, J.; Capra, S.; Battistutta, D.; Davidson, W.; Ash, S. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin. Nutr. 2005, 24, 998–1004. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Braga, M. Perioperative Immunonutrition in Patients Undergoing Cancer Surgery. Arch. Surg. 1999, 134, 428. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.; Bissolati, M.; Rocchetti, S.; Beneduce, A.; Pecorelli, N.; Di Carlo, V. Oral preoperative antioxidants in pancreatic surgery: A double-blind, randomized, clinical trial. Nutrition 2012, 28, 160–164. [Google Scholar] [CrossRef]
- Brennan, M.F.; Pisters, P.W.T.; Posner, M.; Quesada, O.; Shike, M. A Prospective Randomized Trial of Total Parenteral Nutrition After Major Pancreatic Resection for Malignancy. Ann. Surg. 1994, 220, 436–444. [Google Scholar] [CrossRef]
- Daly, J.M.; Weintraub, F.N.; Shou, J.; Rosato, E.F.; Lucia, M. Enteral Nutrition During Multimodality Therapy in Upper Gastrointestinal Cancer Patients. Ann. Surg. 1995, 221, 327–338. [Google Scholar] [CrossRef]
- Douglass, H.O.; Milliron, S.; Nava, H.; Eriksson, B.; Thomas, P.; Novick, A.; Holyoke, E.D. Elemental Diet as an Adjuvant for Patients with Locally Advanced Gastrointestinal Cancer Receiving Radiation Therapy: A Prospectively Randomized Study. JPEN J. Parenter. Enter. Nutr. 1978, 2, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Von Meyenfeldt, M.F.; Moses, A.G.W.; van Geenen, R.; Roy, A.; Gouma, D.J.; Giacosa, A.; van Gossum, A.; Bauer, J.; Barber, M.D.; et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: A randomised double blind trial. Gut 2003, 52, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gade, J.; Levring, T.; Hillingsø, J.; Hansen, C.P.; Andersen, J.R. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay after Elective Surgery for Pancreatic Cancer—A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, C.; Colatruglio, S.; Valoriani, F.; Mazzaferro, V.; Sabbatini, A.; Biffi, R.; Mariani, L.; Miceli, R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: A multicentre randomised clinical trial. Eur. J. Cancer (Oxf. Engl. 1990) 2016, 64, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Büchler, M.; Farhadi, J.; Berger, D.; Hüsler, J.; Schneider, H.; Krähenbühl, S.; Krähenbühl, L. Preoperative Immunonutrition Suppresses Perioperative Inflammatory Response in Patients with Major Abdominal Surgery—A Randomized Controlled Pilot Study. Ann. Surg. Oncol. 2007, 14, 2798–2806. [Google Scholar] [CrossRef]
- Hamza, N.; Darwish, A.; O’Reilly, D.A.; Denton, J.; Sheen, A.J.; Chang, D.; Sherlock, D.J.; Ammori, B.J. Perioperative Enteral Immunonutrition Modulates Systemic and Mucosal Immunity and the Inflammatory Response in Patients with Periampullary Cancer Scheduled for Pancreaticoduodenectomy. Pancreas 2015, 44, 41–52. [Google Scholar] [CrossRef]
- Hyltander, A.; Bosaeus, I.; Svedlund, J.; Liedman, B.; Hugosson, I.; Wallengren, O.; Olsson, U.; Johnsson, E.; Kostic, S.; Henningsson, A.; et al. Supportive Nutrition on Recovery of Metabolism, Nutritional State, Health-Related Quality of Life, and Exercise Capacity after Major Surgery: A Randomized Study. Clin. Gastroenterol. Hepatol. 2005, 3, 466–474. [Google Scholar] [CrossRef]
- Jo, S.; Choi, S.-H.; Heo, J.-S.; Kim, E.-M.; Min, M.-S.; Choi, D.-W.; Seo, J.-M.; Chung, J.-C.; Kim, Y.-I. Missing Effect of Glutamine Supplementation on the Surgical Outcome after Pancreaticoduodenectomy for Periampullary Tumors: A Prospective, Randomized, Double-blind, Controlled Clinical Trial. World J. Surg. 2006, 30, 1974–1982. [Google Scholar] [CrossRef]
- Klek, S.; Kulig, J.; Sierzega, M.; Szczepanek, K.; Szybiński, P.; Scislo, L.; Walewska, E.; Kubisz, A.; Szczepanik, A.M. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery—A prospective, randomized, controlled clinical trial. Clin. Nutr. 2008, 27, 504–512. [Google Scholar] [CrossRef]
- Klek, S.; Sierzega, M.; Szybinski, P.; Szczepanek, K.; Scislo, L.; Walewska, E.; Kulig, J. The immunomodulating enteral nutrition in malnourished surgical patients—A prospective, randomized, double-blind clinical trial. Clin. Nutr. 2011, 30, 282–288. [Google Scholar] [CrossRef]
- Krüger, J.; Meffert, P.J.; Vogt, L.J.; Gärtner, S.; Steveling, A.; Kraft, M.; Mayerle, J.; Lerch, M.M.; Aghdassi, A.A. Early Parenteral Nutrition in Patients with Biliopancreatic Mass Lesions, a Prospective, Randomized Intervention Trial. PLoS ONE 2016, 11, e0166513. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.N.; Williams, R.N.; Welch, N.T.; Aloysius, M.M.; Nunes, Q.M.; Padmanabhan, J.; Crowe, J.R.; Iftikhar, S.Y.; Parsons, S.L.; Neal, K.R.; et al. Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: A prospective, randomized, controlled, double-blind study. Clin. Nutr. 2006, 25, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Matsuyama, R.; Taniguchi, K.; Goto, K.; Miyake, K.; Hiratani, S.; Homma, Y.; Ohta, Y.; Kumamoto, T.; Morioka, D.; et al. Efficacy of prolonged elemental diet therapy after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A pilot prospective randomized trial (UMIN000004108). Clin. Nutr. ESPEN 2019, 34, 116–124. [Google Scholar] [CrossRef]
- Moses, A.W.; Slater, C.; Preston, T.; Barber, M.D.; Fearon, K.C. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br. J. Cancer 2004, 90, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chung, H.-K.; Hwang, H.K.; Kim, J.K.; Yoon, D.S. Postoperative Nutritional Effects of Early Enteral Feeding Compared with Total Parental Nutrition in Pancreaticoduodectomy Patients: A Prosepective, Randomized Study. J. Korean Med. Sci. 2012, 27, 261. [Google Scholar] [CrossRef] [Green Version]
- Perinel, J.; Mariette, C.; Dousset, B.; Sielezneff, I.; Gainant, A.; Mabrut, J.-Y.; Bin-Dorel, S.; Bechwaty, M.E.; Delaunay, D.; Bernard, L.; et al. Early Enteral Versus Total Parenteral Nutrition in Patients Undergoing Pancreaticoduodenectomy. Ann. Surg. 2016, 264, 731–737. [Google Scholar] [CrossRef]
- Slotwinski, R.; Waldemar, L.O.; Lech, G.; Gulak, G.; Sylwia, M.S. Immunonutrition after major pancreatic surgery. Cent. Eur. J. Immunol. 2008, 33, 68. [Google Scholar]
- Werner, K.; Küllenberg de Gaudry, D.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil—A randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef] [Green Version]
- Keum, J.; Chung, M.J.; Kim, Y.; Ko, H.; Sung, M.J.; Jo, J.H.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; et al. Usefulness of Smartphone Apps for Improving Nutritional Status of Pancreatic Cancer Patients: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e21088. [Google Scholar] [CrossRef]
- Amano, K.; Maeda, I.; Ishiki, H.; Miura, T.; Hatano, Y.; Tsukuura, H.; Taniyama, T.; Matsumoto, Y.; Matsuda, Y.; Kohara, H.; et al. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin. Nutr. 2021, 40, 1168–1175. [Google Scholar] [CrossRef]
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Reference | Type of Cancer and Sample Size | Patients’ Characteristics | Intervention | Intervention Time Point/ Duration | Primary Outcome(s) |
|---|---|---|---|---|---|
| Akita et al. (2019) [45] | PaCa n = 62 | IG (n = 31): ♂11; ♀20 67.8 (±10.7) years 22.3 (±2.39) kg/m² CG (n = 31): ♂16; ♀15 66.4 (±9.8) years 22.0 (±3.06) kg/m² | IG: normal diet + EPA-enriched EN as food supplement and 3 nutritional consultations (before, during and after radiation). Composition (target): 2 bottles (440 mL): 560 kcal + EPA (Prosure® (Abbott, Japan))/d. CG: Normal diet. | During neoadjuvant chemoradiotherapy (approx. 5 weeks) | Ratio of skeletal muscle mass |
| Ashida et al. (2019) [46] | Periampullary cancer n = 20 | IG: (n = 11): ♂5; ♀6 64 (±11) years 55.9 (±13.5) kg 22.3 (±7.6) kg/m² CG: (n = 9): ♂6; ♀3 69 (±6) years 56.3 (±7.2) kg 21.4 (±2.5) kg/m² | IG: dietary supplement (target: 600 kcal/d) with EPA fortified diet (2.0 g/d) + regular diet (1200 kcal). CG: standard diet: isocaloric, isonitrogenous standard diet (target: 600 kcal/d) without EPA + regular diet (1200 kcal). | 7 days preOP | Serum concentration of IL-6 |
| Bauer et al. (2005) [47] | PaCa n = 185 | IG: (n = 87): ♂n.a.; ♀n.a. 66.8 (±1.0) years 62.9 (±1.2) kg 22.4 (±0.4) kg/m² CG: (n = 98): ♂n.a.; ♀n.a. 68.3 (±1.1) years 59.3 (±1.3) kg 21.2 (±0.4) kg/m² | IG: target 2 doses of a dietary supplement high in protein and energy + omega-3 fatty acids (1.2 g EPA). CG: isocaloric, isonitrogenous control supplement without omega-3 fatty acids. Both formulas: 310 kcal, 16 g protein. Daily intake Ø 1.5 doses of oral suppl./d (--> 465 kcal and 24 g protein). | Unresectable PaCa; 4–8 weeks | Body composition (body weight, lean body mass) |
| Bourdel-Marchasson et al. (2014) [48] | Mixed n = 336 thereof PaCa n = 62 | IG: (n = 169): ♂81; ♀88 77.7 (±5.2) years WL: 8.9 (±6.6)% CG: (n = 167): ♂91; ♀76 78.3 (±4.7) years WL: 8.6 (±7.9)% | IG: usual care + NI: usual nutrient supply + nutritional counselling. Energy target: 30 kcal/kg body weight/d. Protein target: 1.2 g/kg body weight/d. Possibly (if necessary) dietary supplement. CG: usual care group: normal nutrient supply everything allowed. | According to duration of chemotherapy; 3–4 months | 1-year mortality |
| Braga et al. (1999) [49] | CoCa, GaCa, PaCa n = 171 thereof PaCa n = 22 | IG: (n = 85): ♂50; ♀35 60.9 (±11.9) years 65.8 (±10.9) kg CG: (n = 86): ♂56; ♀30 60.8 (±9.7) years 67.6 (±11.2) kg | IG: EN (Impact, Novartis) (1 L/d) (target): 12.5 g arginine, 1.2 g RNA, 3.3 g omega-3 fatty acids. CG: similar EN without enrichments Both: isocaloric and isonitrogenic. | 6 h postOP–7 days postOP | Rate of postoperative infectious complications and LOS |
| Braga et al. (2012) [50] | PaCa, periampullary cancer n = 36 thereof PaCa n not reported | IG: (n = 18): ♂11; ♀7 64.1 (±10.8) years 25.9 (±4.4) kg/m² WL: 4.4% CG: (n = 18): ♂12; ♀6 64.1 (±12.6) years 24.2 (±3.8) kg/m² WL: 4.3% | IG: dietary supplement as pre-conditioned oral carbohydrate supplement (pONS) enriched with glutamine, antioxidants and green tea extract. Target: 3 doses (first 1 day before surgery at 3 pm, second 6 h later, third on the day of surgery 3 h before induction of anaesthesia); pONS was administered shortly before surgery to have glutamine and antioxidants ready for surgery. CG: Placebo drink. | 1 day preOP–3 h preOP | Postoperative host’s antioxidant capacity (TEAC) and inflammatory response (CRP) |
| Brennan et al. (1994) [51] | PaCa, periampullary cancer n = 117 thereof PaCa n not reported | IG: (n = 60): ♂34; ♀26 65 (34–86) years WL: 5.8 (0–18)% CG (n = 57): ♂27; ♀29 63 (30–86) years WL: 6.8 (0–22)% | IG: total PN 1 day postOP until day with oral intake >1000 kcal/d (12.3 (6–34) d). Total PN (target): 1 g/kg BW/d protein and 30–35 kcal/kg/d + electrolytes, vitamins, minerals (non-protein energy from 70% glucose, 30% fat). CG: dextrose-containing saline until postoperative intake exceeds 1000 kcal/d (22.2 (3–69) d). | 1 day postOP until oral intake >1000 kcal/d | Generic role of total PN (postOP mortality and morbidity) |
| Daly et al. (1995) [52] | OeCa, GaCa, PaCa, others n = 60 thereof PaCa n = 15 | IG1 and 2: (n = 30): ♂25; ♀5 61 (±12) years WL: n = 12 CG 1 and 2:(n = 30): ♂16; ♀14 61 (±10) years WL: n = 10 | IG1 (n = 18): enteral IN in hospital and ambulant. IG 2: (n = 12): enteral IN only in hospital. CG 1: (n = 19): EN with standard diet in hospital and ambulant. CG 2: (n = 11): enteral standard diet only in hospital. Patients did not receive oral nutrition for the first 7 days postOP. | 1 day postOP –12–16 weeks after diagnosis | Clinical outcome, white blood cell fatty acid composition and PGE2 secretion |
| Douglass et al. (1978) [53] | PaCa, GaCa, CoCa n = 30 thereof PaCa n = 15 | No characteristics: n = 13 PaCa; n = 2 Ampullary or Duodenal Ca; n = 5 GaCa n = 5 Rectosigmoidal Ca; n = 4 RectalCa; n = 1 AnalCa | IG: standard diet + dietary supplement (3 times/d) (300 mL of chilled flavoured solution (1 kcal/mL)/ d: 900 kcal. CG: standard diet. | Before planned radiotherapy; between meals three times/d | Weight loss and weight changes |
| Fearon et al. (2003) [54] | PaCa n = 200 | IG: (n = 95): ♂54; ♀41 67 (±1) years 60.3 (±1.1) kg 21.8 (±0.4) kg/m² WL: 17.9 (±0.9)% CG: (n = 105): ♂56; ♀49 68 (±1) years 61.4 (±1.2) kg 22.0 (±0.4) kg/m² WL: 17.1 (±0.8)% | IG: target of 2 doses of dietary supplement with 310 kcal each, 16 g protein, 6 g fat with 1.1 g EPA and antioxidants. CG: target of 2 doses of dietary supplement with 310 kcal each, 16 g protein, 6 g fat and antioxidants. | Advanced PaCa (unresectable) with weight loss; 8 weeks | Body weight and body composition |
| Gade et al. (2016) [55] | PaCa n = 35 | IG: (n = 19): ♂7; ♀12 68 (50–81) years 70.5 (50.8–103.4) kg 24.3 (18.8–28.3) kg/m² WL total: −5.5% (−16.5–2.1) WL last Month: 1.9% (−9.4–2.1) CG: (n = 16): ♂10; ♀6 69 (53–79) years 70.5 (47.5–95.9) kg 23.8 (18.1–30.8) kg/m² WL total: −7.9% (−33.0–3.1) WL last month: 3.95% (−12.9–3.1) | IG: oral EN as IN with target of 1.5 g protein/kg (per pack 16.8 g protein + 250 mL water) between meals (target). Consumption amounts should be recorded in diaries. Recording of other protein intake one week before via Questionnaire on consumption frequency. Estimated dosage (1–4 packs/d) (250–1000 mL: 16.8–67.2 g protein/d) (median intake at 2 pck./d). CG: standard of clinical care (screening with NRS-2002; individual counselling by nursing staff on the topic of food supplements and visit of a nutritionist before diagnosis). | 7 days preOP | Overall postoperative complications and LOS |
| Gavazzi et al. (2016) [56] | OeCa, GaCa, PaCa, bile duct cancer n = 79 thereof PaCa n = 13 | IG: (n = 38): ♂23; ♀15 67 (62–74) years CG: (n = 41): ♂26; ♀15 69 (58–76) years | IG: HEN according to energy requirements by Harris and Benedict. Overnight supplementation to oral diet with any polymeric standard diet containing 1–1.5 kcal/mL, 50–60% carb, 25–35% lipids, 12–20% proteins (target). HEN could be discontinued 2 months after surgery if 5% weight gain was recorded. CG: nutritional counselling by a dietician incl. total energy and protein requirements. If necessary, prescription of oral food supplements. | During oncologic treatment; 1 day postOP– regular oral intake. | Nutritional status (body weight, weight change) |
| Giger et al. (2007) [57] | GaCa, PaCa, periampullary cancer n = 46 thereof PaCa n = 30 | IG1 (n = 14): ♂8; ♀6 64.4 (30–84) years 23.7 (±3.5) kg/m² IG 2 (n = 17): ♂10; ♀7 57.1 (33–77) years 23.3 (±4.0) kg/m² CG (n = 15): ♂9; ♀6 63.0 (47–79) years 22.7 (±3.3) kg/m² | IG1: enteral IN (Impact® (Novartis Consumer Health, Switzerland)) 5 days (1 L/d). IG 2: enteral IN + dietary supplement glycine (Impact plus glycine® (Novartis Consumer Health, Switzerland)) 2 days. CG: no preoperative nutritional support. IG 1 and IG 2: received enteral IN for 7 days postOP, diet should provide 25 kcal/kg/d (target). | 5–2 days preOP IN 1–7 days postOP suppl. or EN + suppl. | Postoperative serum level of C-reactive protein |
| Hamza et al. (2015) [58] | Periampullary cancer n = 37 | IG: (n = 17): ♂9; ♀8 63 (58–69) years WL: 9.2 (6.8–11.6)% CG: (n = 20): ♂11; ♀9 67 (63–70) years WL: 9.6 (7.5–11.8)% | IG: EN: immune-boosting nutrition: Impact® (oral) (Novartis Medical Nutrition, UK) with arginine, omega-3 fatty acids, mRNA. Both: provide 150 kcal/100 mL, non-isonitrogenous. Protein content Impact: 8.4 g/100 mL vs. 6.0 g/100 mL in standard diet due to addition of arginine and mRNA in Impact diet. CG: preOP EN with Fresubin® (Fresenius Kabi Ltd., UK). | 14 days preOP; 24 h to min. 7 days postOP | Parameters of systematic immune function (IL-1-α, TNF-α, total lymphocyte count (TLC), CD4, CD8, CD25, CD56, CH50, C3, C4) |
| Hyltander et al. (2005) [59] | OeCa, GaCa, PaCa, others n = 80 thereof PaCa n not reported | IG1 (n = 26): ♂18; ♀8 62 (±2) years 23.6 (±0.6) kg/m² WL: 5 (±0.4)% IG 2 (n = 27): ♂17; ♀10 62 (±2) years 23.8 (±0.6) kg/m² WL: 5 (±0.3)% CG (n = 27): ♂19; ♀9 63 (±2) years 23.8 (±0.5) kg/m² WL: 5 (±0.8)% | IG1: EN + oral nutrition. IG 2: PN + oral nutrition. CG: oral nutrition. Composition of EN + PN(%kcal): 35 (±3)% fat; 39 (±4)% carb; 16 (±2)% protein; protein 0.9–1.1 g/kg BW/d. Pre- and postOP: counselling by dietician with implementation advice on energy intake, frequency of meals, liquid and solid foods. Oral nutritional supplements were offered (high energy and high protein were recommended). IG 1: Impact® (Novartis Nutrition) (1–10 days postOP). From 11 days postOP standard enteral formula with 1000 mL/d (Nutridrip standard® (Novartis Nutrition)). IG 2: Vitrimix® (Fresenius Kabi) (900 kcal) incl. vitamins, minerals and trace elements CG: standard electrolyte solution. All patients in all groups received recommendations from nutritionists. | 1–10 days postOP–EN; then EN with standard formula. Pre- and post-discharge: nutritional counselling | Recovery of nutritional state (body fat, lean body mass) |
| Jo et al. (2006) [60] | Periampullary cancer n = 60 | IG (n= 32): ♂19; ♀13 56.8 (±9.4) years WL: 3.2 (0–20.5)% CG (n = 28): ♂10; ♀18 56.9 (±10.3) years WL: 5.9 (0–13.4)% | IG: PN as amino acid suppl. + glutamine (2.0 g/100 mL; 15% amino acid solution; target: 10 mL = 0.2 g glutamine/kg BW/d). CG: PN isonitrogenous (target: 1.3 g/kg BW amino acids/d); postOP supplemental (30 kcal/kg/d with 1.3 g/kg amino acids). | Day 2 preOP–5 days postOP | Patient’s discharge from hospital |
| Klek et al. (2008) [61] | GaCa, PaCa, n = 183 thereof PaCa n = 69 | IG: (n = 92): ♂34; ♀14 62.3 (±11.3) years BMI <19 kg/m²: n = 17 BMI >19 kg/m²: n = 74 WL <10% (last 3–6 month): n = 73 WL >10% (last 3–6 month): n = 16 CG: (n = 91): ♂35; ♀13 62.1 (±10.9) years BMI <19 kg/m²: n = 10 BMI >19 kg/m²: n = 38 WL <10% (last 3–6 month): n = 76 WL >10% (last 3–6 month): n = 18 | IG: enteral IN: Reconvan® (Fresenius Kabi, Poland). CG: EN standard oligopeptide diet Peptisorb® (Nutricia Ltd., Poland). Energy same; protein target (CG: 4.0 g vs. IG 5.5 g); total fat target (CG: 1.7 g vs. IG: 4.1 g) (SAFA: 1.0 vs. 3.3; of which MCT: 0.8 vs. 1.9); Sodium: 100 mg vs. 138; Potassium: 150 mg vs. 207. CG: standard oligopeptic EN. | 6 h postOP–7 days postOP | Postoperative infectious complications |
| Klek et al. (2011) [62] | PaCa, GaCa, n = 305 thereof PaCa n = 94 | IG: (n = 152): ♂92; ♀60 60.2 (±12.4) years 17.9 (±2.8) kg/m² WL: 18.3 (±4.4)% CG: (n = 153): ♂89; ♀62 [sic] 61.5 (±11.8) years 17.9 (±2.8) kg/m² WL: 18.8 (±4.9)% | 6 h after surgery with 5% glucose solution for the first 12 h, following. IG: postOP enteral IN: Reconvan®” (Fresenius Kabi, Poland). CG: standard oligopeptide diet: infusion of Peptisorb® (Nutricia Ltd. Poland). | 6 h postOP until 7 days postOP | Number of complications |
| Krueger et al. 2016 [63] | Biliopancreatic lesions partly as PaCa n = 100 | IG: (n = 51): ♂28; ♀23 69.5 (58.2–75.8) years 80.6 (69.8–87.8) kg 26.6 (24.3–32.0) kg/m² WL b. d.: 3.0 (12.0–0.0) kg CG: (n = 49): ♂29; ♀20 61.5 (55.6–71.3) years 75.6 (65.0–85.0) kg 25.3 (22.4–27.8) kg/m² WL b. d.: 5.0 (8.2–0.0) kg | IG: 1000 mL PN (target: 700 kcal, 25.3 g protein, 30 g fat, 75 g glucose + adapted nutrition with vitamins, trace elements on fasting days). CG: 1000 mL isotonic electrolyte solution on fasting days. Same daily oral energy intake during non-fasting days in hospital in CG and IG (1049 vs. 1082 kcal). Median suppl. PN (IG): 1400 kcal. | Undergoing in-hospital work-up for biliopancreatic mass lesions on fasting days | Body weight/ weight gain |
| Lobo et al. (2006) [64] | OeCa, GaCa, PaCa, n = 108 thereof PaCa n = 15 | IG: (n = 54): ♂40; ♀14 65.7 (±1.4) years BMI <19 kg/m²: n = 4 BMI >19 kg/m²: n = 50 CG: (n = 54): ♂43; ♀11 66.6 (±1.4) years BMI <19 kg/m²: n = 5 BMI >19 kg/m²: n = 49 | IG: experimental enteral IN. CG: isonitrogenous, isocaloric standard EN. | 4 h postOP–10–15 days postOP | Development of infectious complications |
| Mori et al. (2019) [65] | PaCa n = 39 | IG: (n = 19): ♂11; ♀8 66 (41–83) years 20.4 (15.0–26.2) kg/m² CG: (n = 20): ♂12; ♀8 64 (41–83) years 20.2 (17.7–29.9) kg/m² | Fat-free elemental EN (via jejunostomy tube). IG: EN until 3 months postOP (91 days (87–93)). CG: until adequate oral intake was achieved (10 days (8–45)). Composition: EN with target 600 kcal/d (2 doses in total) Elental® (EA Pharma Co., Ltd., Japan) with 4.4 g protein/ 100 Kcal. | 1 day postOP–3 months postOP | Complications necessitating readmission (postOP) |
| Moses et al. (2004) [66] | PaCa n = 24 | IG: (n = 9): ♂6; ♀3 65 (±2) years 21 (±1) kg/m² WL: 21 (±2)% CG: (n = 15): ♂4; ♀11 70 (±3) years 20 (±1) kg/m² WL: 19 (±2)% | IGandCG: dietary supplement 8 weeks: 2 doses of 237 mL each containing 16 g protein, 311 kcal (target), both oral supplements ready-to-use, high calorie, high protein, low fat formulas which were isocaloric and isonitrogenic. IG: with omega-3 fatty acids (1.1 g EPA). CG: without omega-3 fatty acids; omega-3 fatty acids: balanced by omega-9 fatty acids. | Home-living PaCa patients (unresectable); 8 weeks | Body weight/ body composition |
| Park et al. (2012) [67] | PaCa, periampullary cancer n = 38 thereof PaCa n = 14 | IG (n = 18): ♂7; ♀11 62.7 (±10.3) years 63.6 (±9.2) kg 23.8 (±3.9) kg/m² WL: 3.1 (±3.6) kg CG (n = 20): ♂12; ♀8 61.3 (±13.2) years 62.7 (±8.5) kg 23.5 (±2.1) kg/m² WL: 1.9 (±1.4) kg | IG: EN: target of 25 kcal/kg (18 h/d). CG: total PN: target of 25 kcal/kg/d. Solution ratio: glucose to lipids 2:1; non-protein to nitrogen (kcal/kg): 100:1. Total PN with vitamins, electrolytes, trace elements, insulin. 4 and 5 days postOP a sip of water. Within 7 days intake of a regular diet. | IG: 24 h postOP-oral intake >800 kcal/d CG: 1 day postOP-oral intake >800 kcal/ d | Change in weight |
| Perinel et al. (2016) [68] | PaCa n = 204 | IG(PN) (n = 101): ♂40; ♀61 64.02 (±9.90) years 23.76 (±3.44) kg/m² WL: 7.31 (±6.81)% CG (EN) (n = 103): ♂39; ♀64 65.46 (±11.25) years 24.99 (±4.17) kg/m² WL: 6.15 (±6.81)% | PreOP IN for all malnourished patients. IG: PN. CG: EN: isonitrogenous, isocaloric feeding (nasojejunale tube) at 25 mL/h from 1 day postOP. Amount increased by 25 mL/h every 24 h (administration over 20 h). IG and CG target: 30 kcal/kg/d with 1.5 g amino acids/kg/d. Carb-/amino acid-ratio: 3/2. | 1 day postOP-oral food intake 60% of nutrient requirement | Incidence of postoperative complications |
| Slotwinski et al. (2008) [69] | PaCa n = 41 | IG: (n = 19): ♂14; ♀5 59.8 (±6.0) years BMI preOP: 23.4 (±4.5) kg/m² BMI postOP: 22.4 (±6.3) kg/m² WL preOP: 6.5 (±2.1)% WL postOP: 9.1 (±2.8)% CG: (n = 22): ♂15; ♀7 54.2 (±4.1) years BMI preOP: 22.2 (±3.2) kg/m² BMI postOP: 21.8 (±3.0) kg/m² WL preOP: 6.3 (±3.4)% WL postOP: 9.2 (±3.2)% | IG: enteral IN: target: 14.7(±2.2)g nitrogen, 177(±26 g) glucose, 51.4(±7.5)g fat, 16.4(±2.4)g glutamine, 10.9(±1.6)g arginine (incl. 91.8(±13.5)g protein and 1529(±224) kcal). CG: standard EN: target: 10.8(±1.3) g nitrogen, 208(±24) g glucose, 66.0(±7.7) g fat (incl. 102(±12) g protein and 1693(±198) kcal). IG+CG: antibiotic as well as low-particle heparin, crystalline-line fluids intravenous and electrolytes as needed. | 1–12.3 (±2.0) day postOP | Cellular immunity |
| Werner at al. (2017) [70] | PaCa n = 33 | IG: (n = 18): ♂7; ♀11 70.3 (±8.24) years 21.3 (±1.73) kg/m² CG: (n = 15): ♂9; ♀6 71.3 (±7.51) years 23.7 (±4.10) kg/m² | IG: dietary supplement IN: FO capsule: 60% FO, 40% MCT (6.9 g/100 g EPA and 13.6 g/100 g DHA); target: 3 × 1 capsule. CG: dietary supplement IN: MPL capsule: 35% omega-3 fatty acids phospholipids (mainly phospatidylcholine) + 65% neutral lipids (8.5 g/100 g EPA and 12.3 g/100 g DHA); target: 3 × 1 capsule MPL and FO each 300 mg EPA and DHA/d. | During chemo-/radio-/supportive or alternative therapy (palliative and curative); 6 weeks | Change in weight and appetite |
| Reference | No. of Patients | Type of Cancer | Evaluated Outcome(s) | Summary of Results |
|---|---|---|---|---|
| Nutritional status (WL, BIA parameters) | ||||
| Krueger et al., 2016 [63] | 100 | Biliopancreatic lesions partly as PaCa | Body weight/ weight gain |
|
| Complications (infections, mortality, LOS) | ||||
| Brennan et al., 1994 [51] | 117 | PaCa; periampullary cancer | Generic role of TPN |
|
| Jo et al. (2006) [60] | 60 | Periampullary cancer | Patients’ discharge from hospital | Trial discontinued: glutamine solution was no longer available.
|
| Reference | No. of Patients | Type of Cancer | Evaluated Outcome(s) | Summary of Results |
|---|---|---|---|---|
| Nutritional status (WL, BIA parameters) | ||||
| Gavazzi et al. (2016) [56] | 79 | OeCa, GaCa, PaCa, Bile duct cancer | Nutritional status | Study was discontinued due to advantage for IG.
|
| Immunology (Cytokines, CRP, Albumin) | ||||
| Slotwinski et al. (2008) [69] | 41 | PaCa | Cytokines |
|
| Hamza et al. (2015) [58] | 37 | Periampullary cancer | Parameters of systematic immune function (IL-1-α, TNF-α) |
|
| Complications (Infections, mortality, LOS) | ||||
| Braga et al. (1999) [49] | 171 | CoCa, GaCa, PaCa | Rate of postoperative infectious complications |
|
| LOS |
| |||
| Daly et al. (1995) [52] | 60 | OeCa, GaCa, PaCa, others | Clinical outcome |
|
| Klek et al. (2008) [61] | 183 | GaCa, PaCa | Postoperative complications |
|
| Lobo et al. (2006) [64] | 108 | OeCa, GaCa, PaCa | Development of infectious complications |
|
| Klek et al. (2011) [62] | 303 | PaCa, GaCa | Number of complications |
|
| Gade et al. (2016) [55] | 35 | PaCa | Overall postoperative complications |
|
| LOS |
| |||
| Mori et al. (2019) [65] | 39 | PaCa | Complications necessitating readmission postOP |
|
| Reference | No. of Patients | Type of Cancer | Evaluated Outcome(s) | Summary of Results |
|---|---|---|---|---|
| Nutritional status (WL, BIA parameters) | ||||
| Douglass et al. (1978) [53] | 30 | PaCa, GaCa, CoCa | Weight loss/weight changes |
|
| Bauer et al. (2005) [47] | 185 | PaCa | Body composition |
|
| Fearon et al. (2003) [54] | 200 | PaCa | Body weight and body composition |
|
| Moses et al. (2004) [66] | 24 | PaCa | Body weight and body composition |
|
| Werner at al. (2017) [70] | 33 | PaCa | Change in weight |
|
| Immunology (Cytokine, CRP, Albumin) | ||||
| Ashida et al. (2019) [46] | 20 | Periampullary cancer | Serum concentration of IL-6 |
|
| Braga et al. (2012) [50] | 36 | PaCa, periampullary cancer | Inflammatory response |
|
| Reference | No. of Patients | Type of Cancer | Evaluated Outcome(s) | Summary of Results |
|---|---|---|---|---|
| Nutritional status (WL, BIA parameters) | ||||
| Park et al. (2012) [67] | 38 | PaCa, periampullary cancer | Change in weight |
|
| Akita et al. (2019) [45] | 62 | PaCa | Ratio of skeletal muscle mass and psoas major muscle mass |
|
| Hyltander et al. (2005) [59] | 80 | OeCa, GaCa, PaCa, others | Recovery of nutritional state |
|
| Immunology (Cytokines, CRP, Albumin) | ||||
| Giger et al. (2007) [57] | 46 | GaCa, PaCa, periampullary cancer | Postoperative serum level of CRP |
|
| Complications (Infections, Mortality, LOS) | ||||
| Bourdel-Marchasson et al. (2014) [48] | 336 | Colon (22.4%), lymphoma (14.9%), lung cancer (10.4%), abdominal PaCa (17.0%) | 1-year mortality |
|
| Perinel et al. (2016) [68] | 204 | PaCa | Incidence of postoperative complications |
|
| Incidence of infectious complications |
| |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rötzer, I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers 2022, 14, 2212. https://doi.org/10.3390/cancers14092212
Emanuel A, Krampitz J, Rosenberger F, Kind S, Rötzer I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers. 2022; 14(9):2212. https://doi.org/10.3390/cancers14092212
Chicago/Turabian StyleEmanuel, Aline, Julia Krampitz, Friederike Rosenberger, Sabine Kind, and Ingeborg Rötzer. 2022. "Nutritional Interventions in Pancreatic Cancer: A Systematic Review" Cancers 14, no. 9: 2212. https://doi.org/10.3390/cancers14092212
APA StyleEmanuel, A., Krampitz, J., Rosenberger, F., Kind, S., & Rötzer, I. (2022). Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers, 14(9), 2212. https://doi.org/10.3390/cancers14092212