Changes in the Number of Gastrointestinal Cancers and Stage at Diagnosis with COVID-19 Pandemic in Japan: A Multicenter Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Patients and Cancer Registry
2.3. Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
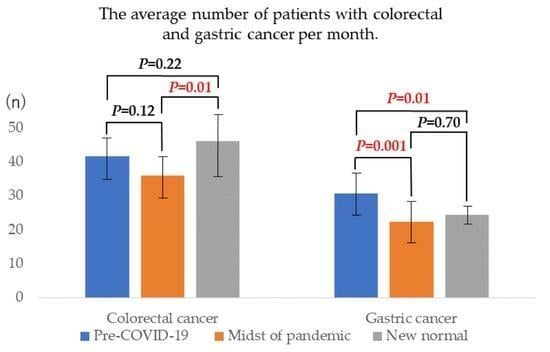
3.2. Colorectal Cancer
3.3. Gastric Cancer
3.4. Pancreatic Cancer
3.5. Esophageal Cancer
3.6. Hepatocellular Carcinoma
3.7. Biliary Tract Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A systematic review. Ann. Intern. Med. 2021, 174, 655–662. [Google Scholar] [CrossRef]
- Czeisler, M.É.; Marynak, K.; Clarke, K.E.N.; Salah, Z.; Shakya, I.; Thierry, J.M.; Ali, N.; McMillan, H.; Wiley, J.F.; Weaver, M.D.; et al. Delay or avoidance of medical care because of COVID-19–Related concerns—United States, June 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1250–1257. [Google Scholar] [CrossRef]
- Fedewa, S.A.; Star, J.; Bandi, P.; Minihan, A.; Han, X.; Yabroff, K.R.; Jemal, A. Changes in cancer screening in the US during the COVID-19 pandemic. JAMA Netw. Open 2022, 5, e2215490. [Google Scholar] [CrossRef]
- Chen, R.C.; Haynes, K.; Du, S.; Barron, J.; Katz, A.J. Association of Cancer Screening Deficit in the United States with the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 878–884. [Google Scholar] [CrossRef]
- Machii, R.; Takahashi, H. Japanese cancer screening programs during the COVID-19 pandemic: Changes in participation between 2017–2020. Cancer Epidemiol. 2023, 82, 102313. [Google Scholar] [CrossRef]
- Lee, K.; Suh, M.; Jun, J.K.; Choi, K.S. Impact of the COVID-19 pandemic on gastric cancer screening in South Korea: Results from the Korean National Cancer screening survey (2017–2021). J. Gastric Cancer 2022, 22, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Star, J.; Bandi, P.; Siegel, R.L.; Han, X.; Minihan, A.; Smith, R.A.; Jemal, A. Cancer screening in the United States during the second year of the COVID-19 pandemic. J. Clin. Oncol. 2023, JCO2202170. [Google Scholar] [CrossRef]
- Lee, J.K.; Lam, A.Y.; Jensen, C.D.; Marks, A.R.; Badalov, J.; Layefsky, E.; Kao, K.; Ho, N.J.; Schottinger, J.E.; Ghai, N.R.; et al. Impact of the COVID-19 pandemic on fecal immunochemical testing, colonoscopy services, and colorectal neoplasia detection in a Large United States community-based population. Gastroenterology 2022, 163, 723–731.e6. [Google Scholar] [CrossRef]
- Ishibashi, F.; Shida, D.; Suzuki, S.; Nagai, M.; Mochida, K.; Morishita, T. A delay in the diagnosis of colorectal cancer screened by fecal immunochemical tests during the COVID-19 pandemic: A longitudinal cohort study. Int. J. Color. Dis. 2022, 37, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Heslin, M.E.; Thau, L.; Smith, A.; Jovin, T.G. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J. Stroke Cerebrovasc. Dis. 2020, 29, 104953. [Google Scholar] [CrossRef]
- Colivicchi, F.; Di Fusco, S.A.; Magnanti, M.; Cipriani, M.; Imperoli, G. The impact of the coronavirus Disease-2019 pandemic and italian lockdown measures on clinical presentation and management of acute heart failure. J. Card. Fail. 2020, 26, 464–465. [Google Scholar] [CrossRef]
- Nopp, S.; Janata-Schwatczek, K.; Prosch, H.; Shulym, I.; Königsbrügge, O.; Pabinger, I.; Ay, C. Pulmonary embolism during the COVID-19 pandemic: Decline in diagnostic procedures and incidence at a university hospital. Res. Pract. Thromb. Haemost. 2020, 4, 835–841. [Google Scholar] [CrossRef]
- Khunti, K.; Valabhji, J.; Misra, S. Diabetes and the COVID-19 pandemic. Diabetologia 2023, 66, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Chen, Z.; Niles, J.; Fesko, Y. Changes in the number of US Patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw. Open 2020, 3, e2017267. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Brookes, M.; Lee, T.J.; Rogers, P.; Sharp, L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: A National Endoscopy Database Analysis. Gut 2021, 70, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef]
- Jacob, L.; Loosen, S.H.; Kalder, M.; Luedde, T.; Roderburg, C.; Kostev, K. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers 2021, 13, 408. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A global challenge with old history, epidemiology and progress so far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef]
- Matta, S.; Chopra, K.K.; Arora, V.K. Morbidity and mortality trends of COVID-19 in top 10 countries. Indian J. Tuberc. 2020, 67, S167–S172. [Google Scholar] [CrossRef]
- Onyeaka, H.; Anumudu, C.K.; Al-Sharify, Z.T.; Egele-Godswill, E.; Mbaegbu, P. COVID-19 pandemic: A review of the global lockdown and its far-reaching effects. Sci. Prog. 2021, 104, 368504211019854. [Google Scholar] [CrossRef]
- Rader, B.; White, L.F.; Burns, M.R.; Chen, J.; Brilliant, J.; Cohen, J.; Shaman, J.; Brilliant, L.; Kraemer, M.U.G.; Hawkins, J.B.; et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: A cross-sectional study. Lancet Digit. Health 2021, 3, e148–e157. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Nguyen, P.-Y.; Chughtai, A.A.; Trent, M.; Gerber, B.; Steinhofel, K.; Seale, H. Mask use, risk-mitigation behaviours and pandemic fatigue during the COVID-19 pandemic in five cities in Australia, the UK and USA: A cross-sectional survey. Int. J. Infect. Dis. 2021, 106, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Kuzuu, K.; Misawa, N.; Ashikari, K.; Kessoku, T.; Kato, S.; Hosono, K.; Yoneda, M.; Nonaka, T.; Matsushima, S.; Komatsu, T.; et al. Gastrointestinal cancer stage at diagnosis before and during the COVID-19 pandemic in Japan. JAMA Netw. Open 2021, 4, e2126334. [Google Scholar] [CrossRef] [PubMed]
- Mentrasti, G.; Cantini, L.; Zichi, C.; D’ostilio, N.; Gelsomino, F.; Martinelli, E.; Chiari, R.; La Verde, N.; Bisonni, R.; Cognigni, V.; et al. Alarming Drop in Early Stage Colorectal Cancer Diagnoses after COVID-19 Outbreak: A Real-World Analysis from the Italian COVID-DELAY Study. Oncologist 2022, 27, e723–e730. [Google Scholar] [CrossRef] [PubMed]
- Doeve, B.H.; Bakx, J.A.C.; Siersema, P.D.; Rosman, C.; van Grieken, N.C.T.; Henegouwen, M.I.V.B.; van Sandick, J.W.; Verheij, M.; Bijlsma, M.F.; Verhoeven, R.H.A.; et al. The impact of the COVID-19 pandemic on the diagnosis, stage, and treatment of esophagogastric cancer. J. Gastroenterol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Geh, D.; Watson, R.; Sen, G.; French, J.J.; Hammond, J.; Turner, P.; Hoare, T.; Anderson, K.; McNeil, M.; McPherson, S.; et al. COVID-19 and liver cancer: Lost patients and larger tumours. BMJ Open Gastroenterol. 2022, 9, e000794. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Levy, M.E.; Natarajan, K.; Reese, S.E.; Naleway, A.L.; Grannis, S.J.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Gaglani, M.; et al. Estimation of COVID-19 mRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status During Omicron BA.4 and BA.5 Sublineage Periods. JAMA Netw. Open 2023, 6, e232598. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Osterholm, M.; Gounder, C.R. A National Strategy for the “New Normal” of Life with COVID. JAMA 2022, 327, 211–212. [Google Scholar] [CrossRef]
- Anzai, A.; Jung, S.-M.; Nishiura, H. Go to Travel campaign and the geographic spread of COVID-19 in Japan. BMC Infect. Dis. 2022, 22, 808. [Google Scholar] [CrossRef]
- Shimoda, H.; Nagata, T.; Ishimaru, T.; Hino, A.; Ando, H.; Muramatsu, K.; Tateishi, S.; Tsuji, M.; Mori, K. Personal infection prevention behaviors and campaign to encourage travel during COVID-19: A cross-sectional study. Front. Public Health 2023, 11, 1037496. [Google Scholar] [CrossRef]
- NHK (Japan Broadcasting Corporation). Number of Infected People in Japan (NHK Summary). Available online: https://www3.nhk.or.jp/news/special/coronavirus/data-all/ (accessed on 29 October 2020).
- Cancer Information Service; National Cancer Center. Japan (Ministry of Health, Labour and Welfare, National Cancer Registry). Cancer Statistics. Available online: https://ganjoho.jp/reg_stat/statistics/index.html (accessed on 28 March 2023).
- Rottoli, M.; Gori, A.; Pellino, G.; Flacco, M.E.; Martellucci, C.; Spinelli, A.; Poggioli, G.; Romano, A.; Belvedere, A.; Lanci, A.L.; et al. Colorectal Cancer Stage at Diagnosis before vs. during the COVID-19 Pandemic in Italy. JAMA Netw. Open 2022, 5, e2243119. [Google Scholar] [CrossRef] [PubMed]
- Cano-Valderrama, O.; Sánchez-Santos, R.; Vigorita, V.; Paniagua, M.; Flores, E.; Garrido, L.; Facal, C.; Ruano, A.; San-Ildefonso, A.; Moncada, E. Has the COVID-19 pandemic changed the clinical picture and tumour stage at the time of presentation of patients with colorectal cancer? A retrospective cohort study. Cir. Esp. 2023, 101, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Schottinger, J.E.; Marks, A.R.; Zhao, W.K.; Ghai, N.R.; et al. Association Between Time to Colonoscopy after a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA 2017, 317, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Beshara, A.; Ahoroni, M.; Comanester, D.; Vilkin, A.; Boltin, D.; Dotan, I.; Niv, Y.; Cohen, A.D.; Levi, Z. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int. J. Cancer 2020, 146, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.; Hilsden, R.J.; Martel, M.; Ruan, Y.; Dube, C.; Rostom, A.; Shorr, R.; Menard, C.; Brenner, D.R.; Barkun, A.N.; et al. Association between Time to Colonoscopy after Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review. Clin. Gastroenterol. Hepatol. 2021, 19, 1344–1354.e8. [Google Scholar] [CrossRef]
- Mutneja, H.R.; Bhurwal, A.; Arora, S.; Vohra, I.; Attar, B.M. A delay in colonoscopy after positive fecal tests leads to higher incidence of colorectal cancer: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1479–1486. [Google Scholar] [CrossRef]
- Johnson, B.A.; Waddimba, A.C.; Ogola, G.O.; Fleshman, J.W., Jr.; Preskitt, J.T. A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: Implication for surgical triage during the COVID-19 pandemic. Am. J. Surg. 2021, 222, 311–318. [Google Scholar] [CrossRef]
- Cone, E.B.; Marchese, M.; Paciotti, M.; Nguyen, D.-D.; Nabi, J.; Cole, A.P.; Molina, G.; Molina, R.L.; Minami, C.A.; Mucci, L.A.; et al. Assessment of Time-to-Treatment Initiation and Survival in a Cohort of Patients with Common Cancers. JAMA Netw. Open 2020, 3, e2030072. [Google Scholar] [CrossRef]
- Tada, M.; Misaki, F.; Kawai, K. Growth rates of colorectal carcinoma and adenoma by roentgenologic follow-up observations. Gastroenterol. Jpn. 1984, 19, 550–555. [Google Scholar] [CrossRef]
- Kodama, M.; Miyamori, D.; Kanno, K.; Ito, M. The impact of early-stage COVID-19 pandemic on the diagnosis and treatment of gastric cancer: A cross-sectional study using a large-scale cancer registry in Hiroshima, Japan. DEN Open 2023, 3, e180. [Google Scholar] [CrossRef]
- Okuyama, A.; Watabe, M.; Makoshi, R.; Takahashi, H.; Tsukada, Y.; Higashi, T. Impact of the COVID-19 pandemic on the diagnosis of cancer in Japan: Analysis of hospital-based cancer registries. Jpn. J. Clin. Oncol. 2022, 52, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; Bencivenga, M.; Rosa, F.; D’ignazio, A.; Marino, E.; Ministrini, S.; Sofia, S.; Sacco, M.; Mura, G.; Rausa, E.; et al. Consequences of the COVID-19 pandemic on the diagnosis and treatment of gastric cancer in referral centers in Italy. Tumori J. 2023, 109, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Sakuramoto, S.; Miyawaki, Y.; Morimoto, Y.; Ebara, G.; Nishibeppu, K.; Oya, S.; Fujihata, S.; Lee, S.; Sugita, H.; et al. Impact of the first era of the coronavirus disease 2019 pandemic on gastric cancer patients: A single-institutional analysis in Japan. Int. J. Clin. Oncol. 2022, 27, 930–939. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Caviglia, G.P.; Gaia, S.; Rolle, E.; Risso, A.; Campion, D.; Brunocilla, P.R.; Saracco, G.M.; Carucci, P. Effect of COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis: Results from a Tertiary Care Center in North-West Italy. Curr. Oncol. 2022, 29, 1422–1429. [Google Scholar] [CrossRef]
- Aljiffry, M.; Abdulelah, A.; Walsh, M.; Peltekian, K.; Alwayn, I.; Molinari, M. Evidence-Based Approach to Cholangiocarcinoma: A Systematic Review of the Current Literature. J. Am. Coll. Surg. 2009, 208, 134–147. [Google Scholar] [CrossRef]
| Pre-COVID-19 a | Phase 1 b | Phase 2 c | p-Value d | |
|---|---|---|---|---|
| Gastrointestinal cancers | ||||
| Total, n | 4218 | 949 | 1286 | |
| Age, mean ± SD | 71.3 ± 10.9 | 71.8 ± 10.7 | 71.9 ± 11.8 | 0.15 |
| Men, n(%) | 2825(67.0%) | 607(64.0%) | 826(64.2%) | 0.07 |
| Women, n(%) | 1393(33.0%) | 342(36.0%) | 460(35.8%) | |
| Colorectal cancer | ||||
| Total, n | 1581 | 360 | 552 | |
| Age, mean ± SD | 70.4 ± 11.6 | 70.8 ± 11.6 | 70.8 ± 12.3 | 0.74 |
| Men, n(%) | 978(61.9%) | 208(57.8%) | 339(61.4%) | 0.36 |
| Women, n(%) | 603(38.1%) | 152(42.2%) | 213(38.6%) | |
| Gastric cancer | ||||
| Total, n | 1164 | 224 | 294 | |
| Age, mean ± SD | 72.5 ± 10.0 | 73.5 ± 9.3 | 73.7 ± 11.4 | 0.10 |
| Men, n(%) | 836(71.8%) | 160(71.4%) | 191(65.0%) | 0.07 |
| Women, n(%) | 328(28.2%) | 64(28.6%) | 103(35.0%) | |
| Pancreatic cancer | ||||
| Total, n | 532 | 141 | 171 | |
| Age, mean ± SD | 69.4 ± 11.5 | 71.0 ± 10.8 | 70.9 ± 12.6 | 0.17 |
| Men, n(%) | 311(58.5%) | 73(51.8%) | 100(58.5%) | 0.34 |
| Women, n(%) | 221(41.5%) | 68(48.2%) | 71(41.5%) | |
| Esophageal cancer | ||||
| Total, n | 335 | 87 | 92 | |
| Age, mean ± SD | 71.6 ± 9.1 | 71.8 ± 10.0 | 71.3 ± 10.7 | 0.94 |
| Men, n(%) | 275(82.1%) | 68(78.2%) | 78(84.8%) | 0.51 |
| Women, n(%) | 60(17.9%) | 19(21.8%) | 14(15.2%) | |
| Hepatocellular carcinoma | ||||
| Total, n | 338 | 75 | 80 | |
| Age, mean ± SD | 73.5 ± 10.8 | 72.5 ± 10.4 | 73.3 ± 11.3 | 0.78 |
| Men, n(%) | 244(72.2%) | 57(76.0%) | 63(78.8%) | 0.44 |
| Women, n(%) | 94(27.8%) | 18(24.0%) | 17(21.2%) | |
| Biliary tract cancer | ||||
| Total, n | 268 | 62 | 97 | |
| Age, mean ± SD | 72.2 ± 10.2 | 72.8 ± 10.6 | 74.1 ± 8.8 | 0.26 |
| Men, n(%) | 181(67.5%) | 41(66.1%) | 55(56.7%) | 0.16 |
| Women, n(%) | 87(32.5%) | 21(33.9%) | 42(43.3%) |
| Background | Number of Patients per Month, Mean (SD) | p-Value a | ||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| First visit | 394(33.8) | 332(88.3) | 365(34.9) | 0.046 * | 0.06 | 0.60 |
| Follow-up visit | 4458(279) | 4128(564) | 4291(382) | 0.31 | 0.37 | 0.97 |
| Gastroscopy | 736(84.2) | 693(125) | 755(69.2) | 0.54 | 0.85 | 0.46 |
| Colonoscopy | 441(45.3) | 399(82.9) | 436(25.6) | 0.42 | 0.96 | 0.79 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Colorectal cancer | ||||||
| Total | 41.61(6.81) | 36.00(6.72) | 46.00(11.32) | 0.12 | 0.22 | 0.01 * |
| Stage 0 | 10.58(3.36) | 7.10(4.09) | 11.00(3.91) | 0.02 * | 0.93 | 0.04 * |
| Stage I | 10.16(3.14) | 6.70(2.91) | 9.42(2.50) | 0.01 * | 0.74 | 0.09 |
| Stage II | 7.42(3.05) | 4.80(1.75) | 6.00(2.86) | 0.03 * | 0.3 | 0.59 |
| Stage III | 7.18(2.85) | 12.10(2.42) | 11.92(3.96) | <0.001 * | <0.001 * | 0.99 |
| Stage IV | 6.26(3.13) | 5.30(2.83) | 7.67(2.99) | 0.65 | 0.36 | 0.18 |
| Medical Checkup cases b | 5.45(2.36) | 4.10(1.91) | 8.75(3.19) | 0.29 | <0.001 * | <0.001 * |
| Screening cases b | 13.84(4.06) | 10.10(2.38) | 16.50(4.01) | 0.02 * | 0.10 | <0.001 * |
| Symptomatic cases b | 20.95(5.05) | 20.50(3.44) | 19.58(7.86) | 0.92 | 0.74 | 0.97 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Gastric cancer | ||||||
| Total | 30.63(6.62) | 22.40(5.85) | 24.50(4.15) | 0.001 * | 0.01 * | 0.70 |
| Stage I | 21.55(5.66) | 13.90(5.99) | 15.75(3.84) | <0.001 * | 0.01 * | 0.71 |
| Stage II | 2.71(1.59) | 2.20(1.4) | 2.17(2.08) | 0.67 | 0.59 | 1.00 |
| Stage III | 1.97(1.37) | 1.40(1.07) | 3.25(2.05) | 0.53 | 0.03 * | 0.01 * |
| Stage IV | 4.39(2.02) | 4.90(3.51) | 3.33(1.5) | 0.80 | 0.33 | 0.24 |
| Medical Checkup cases b | 5.58(2.20) | 3.70(1.34) | 6.42(3.06) | 0.06 | 0.51 | 0.02 * |
| Screening cases b | 12.97(3.61) | 8.00(2.54) | 8.50(2.39) | <0.001 * | <0.001 * | 0.93 |
| Symptomatic cases b | 10.68(3.65) | 10.20(2.82) | 9.25(2.93) | 0.92 | 0.42 | 0.79 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Pancreatic cancer | ||||||
| Total | 14.00(3.37) | 14.10(3.31) | 14.25(4.18) | 1.00 | 0.98 | 0.99 |
| Stage 0 | 0.50(0.60) | 0.80(0.79) | 0.25(0.45) | 0.36 | 0.44 | 0.10 |
| Stage I | 3.34(2.45) | 2.50(1.58) | 3.83(1.70) | 0.53 | 0.78 | 0.34 |
| Stage II | 2.45(1.54) | 2.40(1.71) | 1.67(1.50) | 1.00 | 0.29 | 0.52 |
| Stage III | 2.08(1.58) | 2.30(1.64) | 2.58(2.11) | 0.93 | 0.65 | 0.92 |
| Stage IV | 5.63(2.31) | 6.10(2.51) | 5.92(1.38) | 0.82 | 0.92 | 0.98 |
| Medical Checkup cases b | 1.00(0.99) | 0.80(0.63) | 1.25(0.97) | 0.82 | 0.70 | 0.50 |
| Screening cases b | 4.05(1.77) | 3.70(1.49) | 5.33(2.93) | 0.87 | 0.14 | 0.15 |
| Symptomatic cases b | 8.42(2.97) | 9.00(2.49) | 7.33(2.71) | 0.84 | 0.49 | 0.37 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Esophageal cancer | ||||||
| Total | 8.82(2.75) | 8.70(2.71) | 7.67(3.65) | 0.99 | 0.47 | 0.69 |
| Stage 0 | 1.29(0.96) | 0.90(0.74) | 0.83(0.94) | 0.46 | 0.30 | 0.98 |
| Stage I | 3.16(1.52) | 3.10(1.45) | 2.83(1.90) | 0.99 | 0.81 | 0.92 |
| Stage II | 0.97(1.03) | 0.80(0.79) | 1.33(1.15) | 0.88 | 0.54 | 0.45 |
| Stage III | 1.32(1.23) | 1.50(1.27) | 1.00(1.13) | 0.91 | 0.72 | 0.61 |
| Stage IV | 2.08(1.60) | 2.40(1.43) | 1.67(1.44) | 0.83 | 0.70 | 0.51 |
| Medical Checkup cases b | 0.97(0.94) | 1.10(0.88) | 1.67(1.30) | 0.93 | 0.11 | 0.40 |
| Screening cases b | 3.39(1.57) | 3.00(1.33) | 2.42(1.62) | 0.75 | 0.14 | 0.65 |
| Symptomatic cases b | 3.84(1.84) | 4.00(1.70) | 3.50(2.07) | 0.97 | 0.84 | 0.81 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Hepatocellular carcinoma | ||||||
| Total | 8.89(3.17) | 7.50(2.27) | 6.67(2.61) | 0.38 | 0.07 | 0.79 |
| Stage I | 4.87(2.28) | 3.30(2.06) | 3.58(1.78) | 0.11 | 0.18 | 0.95 |
| Stage II | 1.84(1.72) | 1.70(1.49) | 1.58(1.08) | 0.97 | 0.87 | 0.98 |
| Stage III | 1.34(1.12) | 1.60(1.71) | 0.83(0.72) | 0.81 | 0.40 | 0.29 |
| Stage IV | 0.84(0.92) | 0.90(1.10) | 0.67(0.65) | 0.98 | 0.83 | 0.82 |
| Medical Checkup cases b | 0.55(0.60) | 0.80(0.63) | 0.58(0.67) | 0.50 | 0.99 | 0.69 |
| Screening cases b | 6.55(2.84) | 4.60(1.65) | 4.75(2.14) | 0.09 | 0.09 | 0.99 |
| Symptomatic cases b | 1.55(1.06) | 1.70(1.16) | 1.08(0.90) | 0.92 | 0.37 | 0.36 |
| Number of Patients per Month, Mean (SD) | p-Value a | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Phase 1 | Phase 2 | Pre-COVID-19 vs. Phase 1 | Pre-COVID-19 vs. Phase 2 | Phase 1 vs. Phase 2 | |
| Biliary tract cancer | ||||||
| Total | 7.05(3.06) | 6.20(2.25) | 8.08(3.73) | 0.72 | 0.58 | 0.34 |
| Stage 0 | 0.34(0.58) | 0.30(0.67) | 0.17(0.58) | 0.98 | 0.65 | 0.86 |
| Stage I | 1.29(1.41) | 1.10(0.99) | 0.75(0.87) | 0.91 | 0.41 | 0.79 |
| Stage II | 1.84(1.48) | 2.30(1.64) | 2.25(1.22) | 0.65 | 0.68 | 1.00 |
| Stage III | 1.55(1.37) | 1.20(1.03) | 2.58(1.24) | 0.73 | 0.051 | 0.04 * |
| Stage IV | 2.03(1.64) | 1.30(1.16) | 2.33(2.06) | 0.44 | 0.84 | 0.32 |
| Medical Checkup cases b | 0.45(0.69) | 0.40(0.52) | 0.42(0.90) | 0.98 | 0.99 | 1.00 |
| Screening cases b | 1.92(1.62) | 1.30(1.16) | 2.08(1.08) | 0.47 | 0.94 | 0.43 |
| Symptomatic cases b | 4.42(2.10) | 4.40(1.84) | 5.33(2.81) | 1.00 | 0.43 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzuu, K.; Misawa, N.; Ashikari, K.; Tamura, S.; Kato, S.; Hosono, K.; Yoneda, M.; Nonaka, T.; Matsushima, S.; Komatsu, T.; et al. Changes in the Number of Gastrointestinal Cancers and Stage at Diagnosis with COVID-19 Pandemic in Japan: A Multicenter Cohort Study. Cancers 2023, 15, 4410. https://doi.org/10.3390/cancers15174410
Kuzuu K, Misawa N, Ashikari K, Tamura S, Kato S, Hosono K, Yoneda M, Nonaka T, Matsushima S, Komatsu T, et al. Changes in the Number of Gastrointestinal Cancers and Stage at Diagnosis with COVID-19 Pandemic in Japan: A Multicenter Cohort Study. Cancers. 2023; 15(17):4410. https://doi.org/10.3390/cancers15174410
Chicago/Turabian StyleKuzuu, Kento, Noboru Misawa, Keiichi Ashikari, Shigeki Tamura, Shingo Kato, Kunihiro Hosono, Masato Yoneda, Takashi Nonaka, Shozo Matsushima, Tatsuji Komatsu, and et al. 2023. "Changes in the Number of Gastrointestinal Cancers and Stage at Diagnosis with COVID-19 Pandemic in Japan: A Multicenter Cohort Study" Cancers 15, no. 17: 4410. https://doi.org/10.3390/cancers15174410
APA StyleKuzuu, K., Misawa, N., Ashikari, K., Tamura, S., Kato, S., Hosono, K., Yoneda, M., Nonaka, T., Matsushima, S., Komatsu, T., Nakajima, A., & Higurashi, T. (2023). Changes in the Number of Gastrointestinal Cancers and Stage at Diagnosis with COVID-19 Pandemic in Japan: A Multicenter Cohort Study. Cancers, 15(17), 4410. https://doi.org/10.3390/cancers15174410