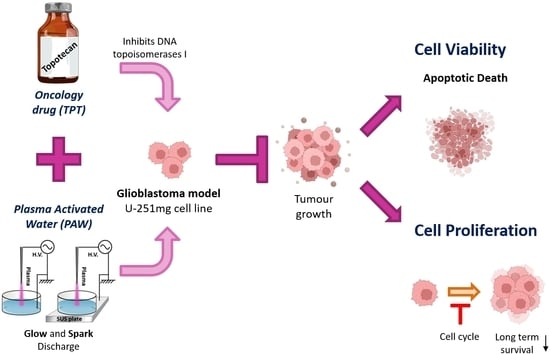
Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup for Plasma Generation
2.2. Plasma-Activated Water Generation and pH Measurements
2.3. Chemical Analysis of Reactive Species in Plasma-Activated Water
2.3.1. Determination of Hydrogen Peroxide (H2O2) and Reactive Oxygen Species (ROS)
2.3.2. Determination of Nitrite () and Nitrate ()
2.4. Cell Culture of Human Glioblastoma Multiforme Cell Lines
2.5. Cytotoxicity Evaluation
2.5.1. Single Treatments
2.5.2. Combined Treatment
2.6. Cell Viability Assays
2.6.1. Resazurin/Alamar Blue Assay
2.6.2. Crystal Violet Staining
2.7. Combination Index
2.8. Evaluation of Cell Death by Flow Cytometry
2.9. Evaluation of Cell Proliferation and DNA Content by Flow Cytometry
2.10. Evaluation of Cell Survival Clonogenic Assay
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition of PAW Is Setup-Dependent and Influences the Cytotoxic Effect on U-251mg Cell Line
3.2. PAW and TPT Combinations Decrease the Survival Rate of Glioblastoma Cells
3.3. PAW and TPT Combinations Have an Antiproliferative Effect in Glioblastoma Cells
3.4. Apoptosis Is the Main Pathway of PAW- and PAW+TPT-Induced Cell Death
3.5. Combinatorial Treatments of PAW and TPT Impact Cell Cycle
3.6. PAW and TPT Combination Treatments Have a Long-Term Antiproliferative Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Bi, J.; Chowdhry, S.; Wu, S.; Zhang, W.; Masui, K.; Mischel, P.S. Altered cellular metabolism in gliomas—An emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer 2020, 20, 57–70. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Barcia, C.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Plasma induced reactive oxygen species-dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Process. Polym. 2022, 19, e2100157. [Google Scholar] [CrossRef]
- van Solinge, T.S.; Nieland, L.; Chiocca, E.A.; Broekman, M.L.D. Advances in local therapy for glioblastoma—Taking the fight to the tumour. Nat. Rev. Neurol. 2022, 18, 221–236. [Google Scholar] [CrossRef]
- Mariappan, A.; Goranci-Buzhala, G.; Ricci-Vitiani, L.; Pallini, R.; Gopalakrishnan, J. Trends and challenges in modeling glioma using 3D human brain organoids. Cell Death Differ. 2021, 28, 15–23. [Google Scholar] [CrossRef]
- Rezaei, S.; Assaran Darban, R.; Javid, H.; Hashemy, S.I. The Therapeutic Potential of Aprepitant in Glioblastoma Cancer Cells through Redox Modification. Biomed Res. Int. 2022, 2022, 8540403. [Google Scholar] [CrossRef]
- Sharon Gabbay, R.; Rubinstein, A. Controlling the release rate of topotecan from PLGA spheres and increasing its cytotoxicity towards glioblastoma cells by co-loading with calcium chloride. Int. J. Pharm. 2021, 602, 120616. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Neira, J.A.; Yun, J.; Alexiades, N.G.; Banu, M.; Englander, Z.K.; Kennedy, B.C.; Ung, T.H.; Rothrock, R.J.; Romanov, A. Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J. Neurosurg. 2021, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Joslin, J.M.; McCall, J.R.; Bzdek, J.P.; Johnson, D.C.; Hybertson, B.M. Aqueous plasma pharmacy: Preparation methods, chemistry, and therapeutic applications. Plasma Med. 2016, 6, 135–177. [Google Scholar] [CrossRef]
- Sersenová, D.; Machala, Z.; Repiská, V.; Gbelcová, H. Selective apoptotic effect of plasma activated liquids on human cancer cell lines. Molecules 2021, 26, 4254. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Controlled cytotoxicity of plasma treated water formulated by open-air hybrid mode discharge. Appl. Phys. Lett. 2017, 110, 264102. [Google Scholar] [CrossRef]
- Chen, Z.; Simonyan, H.; Cheng, X.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef]
- Ng, S.W.; Slikboer, E.; Dickenson, A.; Walsh, J.L.; Lu, P.; Boehm, D.; Bourke, P. Characterization of an atmospheric pressure air plasma device under different modes of operation and their impact on the liquid chemistry. J. Appl. Phys. 2021, 129, 123303. [Google Scholar] [CrossRef]
- Tsoukou, E.; Delit, M.; Treint, L.; Bourke, P.; Boehm, D. Distinct chemistries define the diverse biological effects of plasma activated water generated with spark and glow plasma discharges. Appl. Sci. 2021, 11, 1178. [Google Scholar] [CrossRef]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Giovannetti, E.; Peters, G.J. Chapter 34 Analysis of Drug Interactions. In Cancer Cell Culture Methods and Protocols; Humana: Totowa, NJ, USA, 2011; Volume 731, pp. 421–434. [Google Scholar] [CrossRef]
- Silva-Teixeira, R.; Laranjo, M.; Lopes, B.; Almeida-Ferreira, C.; Gonçalves, A.C.; Rodrigues, T.; Matafome, P.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F. Plasma activated media and direct exposition can selectively ablate retinoblastoma cells. Free Radic. Biol. Med. 2021, 171, 302–313. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dabholkar, N.; Pal, U.N.; Singhvi, G.; Sharma, N.K.; Puri, A.; Kesharwani, P. Emerging innovations in cold plasma therapy against cancer: A paradigm shift. Drug Discov. Today 2022, 27, 2425–2439. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Almeida, N.D.; Klein, A.L.; Hogan, E.A.; Terhaar, S.J.; Kedda, J.; Uppal, P.; Sack, K.; Keidar, M.; Sherman, J.H. Cold Atmospheric Plasma as an Adjunct to Immunotherapy for Glioblastoma Multiforme. World Neurosurg. 2019, 130, 369–376. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef]
- Lee, C.B.; Seo, I.H.; Chae, M.-W.; Park, J.W.; Choi, E.H.; Uhm, H.S.; Baik, K.Y. Anticancer Activity of Liquid Treated with Microwave Plasma-Generated Gas through Macrophage Activation. Oxidative Med. Cell. Longev. 2020, 2020, 2946820. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Min, T.; Xie, X.; Ren, K.; Sun, T.; Wang, H.; Dang, C.; Zhang, H. Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor. Front. Med. 2022, 9, 884887. [Google Scholar] [CrossRef]
- Motaln, H.; Recek, N.; Rogelj, B. Intracellular responses triggered by cold atmospheric plasma and plasma-activated media in cancer cells. Molecules 2021, 26, 1336. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.P.; Canal, C. Plasma-conditioned liquids as anticancer therapies In Vivo: Current state and future directions. Cancers 2021, 13, 452. [Google Scholar] [CrossRef]
- Molotkov, A.; Carberry, P.; Dolan, M.A.; Joseph, S.; Idumonyi, S.; Oya, S.; Castrillon, J.; Konofagou, E.E.; Doubrovin, M.; Lesser, G.J.; et al. Real-time positron emission tomography evaluation of topotecan brain kinetics after ultrasound-mediated blood–brain barrier permeability. Pharmaceutics 2021, 13, 405. [Google Scholar] [CrossRef]
- El-Gizawy, S.A.; Hedaya, M.A. Comparative brain tissue distribution of camptothecin and topotecan in the rat. Cancer Chemother. Pharmacol. 1999, 43, 364–370. [Google Scholar] [CrossRef]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of Recurrent Malignant Gliomas with Convection-Enhanced Delivery of Topotecan. Neurosurgery 2011, 69, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Galetta, D.; Giotta, F.; Rinaldi, A.; Romito, S.; Brunetti, C.; Silvestris, N.; Colucci, G. Topotecan in the treatment of brain metastases. A phase II study of GOIM (Gruppo Oncologico dell’Italia Meridionale). Anticancer Res. 2006, 26, 2259–2263. [Google Scholar]
- Spinazzi, E.F.; Argenziano, M.G.; Upadhyayula, P.S.; A Banu, M.; A Neira, J.; O Higgins, D.M.; Wu, P.B.; Pereira, B.; Mahajan, A.; Humala, N.; et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: A first-in-patient, single-centre, single-arm, phase 1b trial. Lancet Oncol. 2022, 23, 1409–1418. [Google Scholar] [CrossRef]
- Upadhyayula, P.S.; Spinazzi, E.F.; Argenziano, M.G.; Canoll, P.; Bruce, J.N. Convection enhanced delivery of topotecan for gliomas: A single-center experience. Pharmaceutics 2021, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Bigner, D.D.; Bigner, S.H.; Pontén, J.; Westermark, B.; Mahaley, M.S.; Ruoslahti, E.; Herschman, H.; Eng, L.F.; Wikstrand, C.J. Heterogeneity of Genotypic and Phenotypic Characteristics of Fifteen Permanent Cell Lines Derived from Human Gliomas. J. Neuropathol. Exoerimental Neurol. 1981, 40, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Belot, N.; Rorive, S.; Doyen, I.; Lefranc, F.; Bruyneel, E.; Dedecker, R.; Micik, S.; Brotchi, J.; Decaestecker, C.; Salmon, I.; et al. Molecular Characterization of Cell Substratum Attachments in Human Glial Tumors Relates to Prognostic Features. Glia 2001, 36, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Tran, K.; Chen, Y.; Di Donato, A.T.; Yu, L.; Hu, Y.; Linskey, M.E.; Wang, P.H.; Limoli, C.L.; Zhou, Y.-H. Linking differential radiation responses to glioma heterogeneity. Oncotarget 2014, 5, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.K.; Alghazwni, M.K.; Alharbi, A.S.; Alqurashi, G.G.; Kamal, M.; Alnufaie, S.R.; Alshammari, S.S.; Alshehri, B.A.; Tayeb, R.H.; Bougeis, R.J.M.; et al. Nanoplatform for the Delivery of Topotecan in the Cancer Milieu: An Appraisal of its Therapeutic Efficacy. Cancers 2023, 15, 65. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Soni, V.; Adhikari, M.; Simonyan, H.; Lin, L.; Sherman, J.H.; Young, C.N.; Keidar, M. In vitro and in vivo enhancement of temozolomide effect in human glioblastoma by non-invasive application of cold atmospheric plasma. Cancers 2021, 13, 4485. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Privat-maldonado, A.; Smits, E.; Bogaerts, A. Cold atmospheric plasma increases temozolomide sensitivity of three-dimensional glioblastoma spheroids via oxidative stress-mediated dna damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic effect of cold atmospheric pressure plasma and free or liposomal doxorubicin on melanoma cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Sanz, M.; Ginebra, M.P.; Tornín, J.; Canal, C. Cold atmospheric plasma enhances doxorubicin selectivity in metastasic bone cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]








| Cell Line | IC50 Value | Resazurin | Crystal Violet |
|---|---|---|---|
| U-251mg | Glow (G15) | 30.03% | 19.98% |
| U-251mg | Spark (S5) | 7.69% | 4.98% |
| U-251mg | Topotecan | 0.2579 nM | 0.2453 nM |
| A172 | Topotecan | 0.1903 nM | 0.1401 nM |
| Combination Index | Resazurin | Crystal Violet | ||
|---|---|---|---|---|
| G15+TPT 0.1 | 1.1696 | Slight Antagonism | 1.4212 | Moderate Antagonism |
| G15+TPT 0.2 | 1.3157 | Moderate Antagonism | 1.5627 | Antagonism |
| S5+TPT 0.1 | 1.0498 | Nearly Additive | 1.3801 | Moderate Antagonism |
| S5+TPT 0.2 | 1.1175 | Slight Antagonism | 1.5116 | Antagonism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro Lopes, B.; O’Neill, L.; Bourke, P.; Boehm, D. Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells. Cancers 2023, 15, 4858. https://doi.org/10.3390/cancers15194858
Pinheiro Lopes B, O’Neill L, Bourke P, Boehm D. Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells. Cancers. 2023; 15(19):4858. https://doi.org/10.3390/cancers15194858
Chicago/Turabian StylePinheiro Lopes, Beatriz, Liam O’Neill, Paula Bourke, and Daniela Boehm. 2023. "Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells" Cancers 15, no. 19: 4858. https://doi.org/10.3390/cancers15194858
APA StylePinheiro Lopes, B., O’Neill, L., Bourke, P., & Boehm, D. (2023). Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells. Cancers, 15(19), 4858. https://doi.org/10.3390/cancers15194858