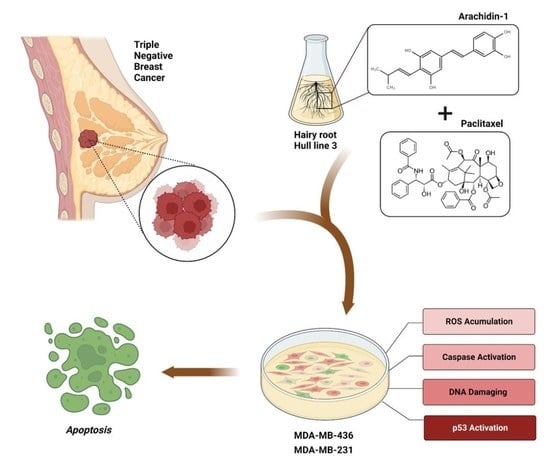
Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Cytotoxicity Assays
2.3. Caspase-3/7 Activity Assays
2.4. Reactive Oxygen Species (ROS)
2.5. Cell Cycle Analyses
2.6. Detection of Apoptosis Using Annexin V-FITC Assays
2.7. Immunoblotting
2.8. 3-Dimensional Culture
2.9. Statistical Analyses
3. Results
3.1. Stilbenoids Increased the Cytotoxicity of Pac in TNBC Cells
3.2. A-1 Combined with Pac Activates Caspase-3/7
3.3. Effect of A-1 Combined with Pac in a Non-Cancerous Cell Line
3.4. Pac Combined with A-1 Induces ROS in TNBC Cells
3.5. A-1 Combined with Pac Induces G2/M Phase Cell Cycle Arrest in TNBC Cells
3.6. Apoptosis Effect of A-1 and Pac alone or in Combination on TNBC Cells
3.7. A-1 Combined with Pac Induced Apoptosis through the Intrinsic Pathway in TNBC Cells
3.8. A-1 Enhances the Efficacy of Pac in TNBC Spheroids by Inducing Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A. Breast cancer statistics: Recent trends. Adv. Exp. Med. Biol. 2019, 1152, 1–7. [Google Scholar]
- Dewangan, J.; Tandon, D.; Srivastava, S.; Verma, A.K.; Yapuri, A.; Rath, S.K. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis 2017, 22, 1246–1259. [Google Scholar] [CrossRef]
- American Cancer Society. Breast Cancer Facts & Figures 2017–2018; American Cancer Society, Inc.: Atlanta, GA, USA, 2018. [Google Scholar]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaou, K.C.; Dai, W.M.; Guy, R.K. Chemistry and biology of taxol. Angew. Chem. Int. Ed. Engl. 1994, 33, 15–44. [Google Scholar] [CrossRef]
- Perez, E.A. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast Cancer Res. Treat. 2008, 114, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Toma, W.; Kyte, S.L.; Bagdas, D.; Alkhlaif, Y.; Alsharari, S.D.; Lichtman, A.H.; Chen, Z.J.; Del Fabbro, E.; Bigbee, J.W.; Gewirtz, D.A. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 2017, 117, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2008, 9, 767–776. [Google Scholar] [CrossRef]
- Mohammadhosseinpour, S.; Clack, B.A. The effect of compound l19 on human colorectal cells (DLD-1). FASEB J. 2017, 31, 774.14. [Google Scholar]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233. [Google Scholar]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Brents, L.K.; Medina-bolivar, F.; Seely, K.A.; Nair, V.; Bratton, S.M.; Ñopo-olazabal, L.; Patel, R.Y.; Liu, H.; Doerksen, R.J.; Prather, P.L.; et al. Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 2012, 42, 139–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.P.; Au, L.C.; Chiou, R.Y.Y.; Chung, P.C.; Chen, S.Y.; Tang, W.C.; Chang, C.L.; Fang, W.H.; Lin, S.B. Arachidin-1, a peanut stilbenoid, induces programmed cell death in human leukemia HL-60 cells. J. Agric. Food Chem. 2010, 58, 12123–12129. [Google Scholar] [CrossRef] [PubMed]
- Witcher, C.; Napier-Jameson, R.; Lockwood, H.; Mattila, M.; Wisdom, S.; Ferreira, L.S.; Taylor, J.; Clack, B.; Medina-Bolivar, F.; Ball, J.; et al. Arachidin-1 and arachidin-3 modulation of rotavirus-infected MA104 cells. J. Med. Act. Plants 2019, 8, 1–19. [Google Scholar]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.C.; Chen, H.J.; Huang, Y.C.; Tseng, S.C.; Weng, S.H.; Wo, T.Y.; Huang, Y.J.; Chiu, H.C.; Tsai, M.S.; Chiou, R.Y.Y.; et al. HSP90 inhibition induces cytotoxicity via down-regulation of Rad51 expression and DNA repair capacity in non-small cell lung cancer cells. Regul. Toxicol. Pharmacol. 2012, 64, 415–424. [Google Scholar] [CrossRef]
- Ko, J.C.; Huang, Y.C.; Chen, H.J.; Tseng, S.C.; Chiu, H.C.; Wo, T.Y.; Huang, Y.J.; Weng, S.H.; Chiou, R.Y.Y.; Lin, Y.W. Metformin induces cytotoxicity by down-regulating thymidine phosphorylase and excision repair cross-complementation 1 expression in non-small cell lung cancer cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 56–65. [Google Scholar] [CrossRef]
- Mohammadhosseinpour, S.; Ho, L.C.; Fang, L.; Xu, J.; Medina-Bolivar, F. Arachidin-1, a prenylated stilbenoid from peanut, induces apoptosis in triple-negative breast cancer cells. Int. J. Mol. Sci. 2022, 23, 1139. [Google Scholar] [CrossRef]
- Fang, L.; Yang, T.; Medina-Bolivar, F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.R.; Gajurel, G.; Ahmed, I.; Roedel, K.; Medina-Bolivar, F. Induction of the prenylated stilbenoids arachidin-1 and arachidin-3 and their semi-preparative separation and purification from hairy root cultures of peanut (Arachis hypogaea l.). Molecules 2022, 27, 6118. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Parra, M.R.; Stoub, H.; Gallo, K.A.; Doseff, A.I. Apigenin by targeting HnRNPA2 sensitizes triple-negative breast cancer spheroids to doxorubicin-induced apoptosis and regulates expression of ABCC4 and ABCG2 drug efflux transporters. Biochem. Pharmacol. 2020, 182, 114259. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2019, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Ying-Jie, K.; Ling-Wei, C.; Min, Z.; Jia-Ping, G.; Ying, W.; Dan, Z.; Sen, Z. Resveratrol enhances chemosensitivity of renal cell carcinoma to paclitaxel. Front. Biosci. 2019, 24, 1452–1461. [Google Scholar]
- Sprouse, A.A.; Herbert, B. Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer. Res. 2014, 34, 5363–5374. [Google Scholar]
- Öztürk, Y.; Günaydın, C.; Yalçın, F.; Nazıroğlu, M.; Braidy, N. Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxid. Med. Cell Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Shen, Y.; Schmidt, B.U.S.; Kubitschke, H.; Morawetz, E.W.; Wolf, B.; Käs, J.A.; Losert, W. Detecting heterogeneity in and between breast cancer cell lines. Cancer Converg. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Kianpour Rad, S.; Kanthimathi, M.S.; Nurestri Abd Malek, S.; Lee, G.S.; Looi, C.Y.; Wong, W.F. Cinnamomum cassia suppresses caspase-9 through stimulation of AKT1 in MCF-7 cells but not in MDA-MB-231 cells. PLoS ONE 2015, 10, e0145216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, G.I.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Invest. 1999, 104, 1645–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatadri, R.; Muni, T.; Iyer, A.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related MiRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinod, B.S.; Nair, H.H.; Vijayakurup, V.; Shabna, A.; Shah, S.; Krishna, A.; Pillai, K.S.; Thankachan, S.; Anto, R.J. Resveratrol chemosensitizes HER-2-Overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis. Cell Death Dis. 2015, 1, 15061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Tao, J.; Zhong, F.; Jiao, Y.; Xu, J.; Shen, Q.; Wang, H.; Fan, S.; Zhang, Y. Polydatin down-regulates the phosphorylation level of creb and induces apoptosis in human breast cancer cell. PLoS ONE 2017, 12, e0176501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar Parida, P.; Mahata, B.; Santra, A.; Chakraborty, S.; Ghosh, Z.; Raha, S.; Misra, A.K.; Biswas, K.; Jana, K. Inhibition of cancer progression by a novel trans-stilbene derivative through disruption of microtubule dynamics, driving G2/M arrest, and P53-dependent apoptosis. Cell Death Dis. 2018, 9, 1–18. [Google Scholar]
- Mao, Q.Q.; Bai, Y.; Lin, Y.W.; Zheng, X.Y.; Qin, J.; Yang, K.; Xie, L.P. Resveratrol confers resistance against taxol via induction of cell cycle arrest in human cancer cell lines. Mol. Nutr. Food Res. 2010, 54, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.; Sreeja, J.S.; Gireesh, K.K.; Gupta, H.; Manna, T.K. +TIP EB1 downregulates paclitaxel-induced proliferation inhibition and apoptosis in breast cancer cells through inhibition of paclitaxel binding on microtubules. Int. J. Oncol. 2015, 46, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Yoo, Y.H. Taxol-induced growth arrest and apoptosis is associated with the upregulation of the Cdk Inhibitor, P21WAF1/CIP1, in human breast cancer cells. Oncol. Rep. 2012, 28, 2163–2169. [Google Scholar] [CrossRef]
- Kim, J.A.H.; Kim, D.H.; Hossain, M.A.; Kim, M.Y.; Sung, B.; Yoon, J.H.; Suh, H.; Jeong, T.C.; Chung, H.Y.; Kim, N.D. HS-1793, a resveratrol analogue, induces cell cycle arrest and apoptotic cell death in human breast cancer cells. Int. J. Oncol. 2014, 44, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Janssen, K.; Pohlmann, S.; Jänicke, R.U.; Schulze-Osthoff, K.; Fischer, U. Apaf-1 and caspase-9 deficiency prevents apoptosis in a Bax-controlled pathway and promotes clonogenic survival during paclitaxel treatment. Blood 2007, 110, 3662–3672. [Google Scholar] [CrossRef]
- Notte, A.; Ninane, N.; Arnould, T.; Michiels, C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: Role of autophagy and JNK activation. Cell Death Dis. 2013, 4, e638. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| 24 h | RES (µM) | Pac IC50 (nM) | Fold Decrease Compared to Pac Alone | Pac (nM) | RES IC50 (µM) | Fold Decrease Compared to RES Alone |
| MDA-MB-231 | 0 | 2.69 ± 0.13 | 0 | 10.67 ± 0.35 | ||
| 0.1 | 1.97 ± 0.25 | 0.96 | 0.1 | 7.94 ± 1.45 | 1.30 | |
| 1 | 1.11 ± 0.23 * | 1.71 | 1 | 6.68 ± 1.34 | 1.55 | |
| 10 | 0.42 ± 0.02 ** | 4.53 | 10 | 6.46 ± 1.24 | 1.60 | |
| MDA-MB-436 | 0 | 2.47 ± 0.28 | 0 | 7.14 ± 2.13 | ||
| 0.1 | 1.59 ± 0.12 | 1.80 | 0.1 | 9.07 ± 0.31 | 1.10 | |
| 1 | 1.20 ± 0.32 * | 2.37 | 1 | 8.52 ± 1.14 | 1.17 | |
| 10 | 0.37 ± 0.04 ** | 7.78 | 10 | 6.16 ± 3.49 | 1.62 | |
| 48 h | RES (µM) | Pac IC50 (nM) | Fold decrease compared to Pac Alone | Pac (nM) | RES IC50 (µM) | Fold decrease compared to RES Alone |
| MDA-MB-231 | 0 | 2.60 ± 0.14 | 0 | 10.36 ± 0.74 | ||
| 0.1 | 1.93 ± 0.32 | 0.98 | 0.1 | 7.39 ± 0.96 | 1.40 | |
| 1 | 0.86 ± 0.19 ** | 2.22 | 1 | 5.14 ± 2.01 ** | 2.01 | |
| 10 | 0.56 ± 0.14 ** | 3.37 | 10 | 9.09 ± 2.55 | 1.14 | |
| MDA-MB-436 | 0 | 2.81 ± 0.44 | 0 | 9.53 ± 0.12 | ||
| 0.1 | 1.54 ± 0.11 | 1.85 | 0.1 | 10.04 ± 0.67 | 0.99 | |
| 1 | 0.90 ± 0.19 ** | 3.17 | 1 | 8.08 ± 2.17 | 1.24 | |
| 10 | 0.61 ± 0.20 ** | 4.64 | 10 | 4.57 ± 1.11 * | 2.19 | |
| 72 h | RES (µM) | Pac IC50 (nM) | Fold decrease compared to Pac Alone | Pac (nM) | RES IC50 (µM) | Fold decrease compared to RES Alone |
| MDA-MB-231 | 0 | 1.90 ± 0.39 | 0 | 10.33 ± 0.64 | ||
| 0.1 | 1.15 ± 0.32 | 1.65 | 0.1 | 10.83 ± 0.92 | 0.95 | |
| 1 | 0.68 ± 0.16 | 2.79 | 1 | 6.65 ± 1.17 | 1.55 | |
| 10 | 0.33 ± 0.01 * | 5.75 | 10 | 11.82 ± 5.30 | 0.87 | |
| MDA-MB-436 | 0 | 2.85 ± 0.17 | 0 | 9.99 ± 1.19 | ||
| 0.1 | 1.82 ± 0.24 | 1.56 | 0.1 | 10.16 ± 0.83 | 0.98 | |
| 1 | 1.40 ± 0.07 | 2.04 | 1 | 9.99 ± 0.54 | 1.00 | |
| 10 | 0.70 ± 0.13 * | 4.06 | 10 | 6.70 ± 1.38 | 1.49 |
| 24 h | A-1 (µM) | Pac IC50 (nM) | Fold decrease Compared to Pac Alone | Pac (nM) | A-1 IC50 (µM) | Fold Decrease Compared to A-1 Alone |
| MDA-MB-231 | 0 | 2.20 ± 0.12 | 0 | 2.71 ± 0.06 | ||
| 0.1 | 1.15 ± 0.11 ** | 1.95 | 0.1 | 1.85 ± 0.38 | 1.80 | |
| 1 | 0.75 ± 0.21 ** | 2.98 | 1 | 0.70 ± 0.27 ** | 4.72 | |
| 10 | 0.39 ± 0.14 ** | 5.72 | 10 | 0.66 ± 0.03 **** | 5.04 | |
| MDA-MB-436 | 0 | 2.24 ± 0.11 | 0 | 2.72 ± 0.14 | ||
| 0.1 | 1.04 ± 0.01 *** | 2.14 | 0.1 | 1.60 ± 0.25 * | 1.34 | |
| 1 | 0.60 ± 0.03 ** | 3.68 | 1 | 0.86 ± 0.34 ** | 2.50 | |
| 10 | 1.78 ± 0.37 * | 1.25 | 10 | 0.45 ± 0.01 **** | 4.78 | |
| 48 h | A-1 (µM) | Pac IC50 (nM) | Fold decrease compared to Pac Alone | Pac (nM) | A-1 IC50 (µM) | Fold decrease compared to A-1 Alone |
| MDA-MB-231 | 0 | 2.21 ± 0.23 | 0 | 2.71 ± 0.17 | ||
| 0.1 | 1.44 ± 0.09 | 1.56 | 0.1 | 1.94 ± 0.36 | 1.72 | |
| 1 | 1.20 ± 0.10 * | 1.87 | 1 | 0.88 ± 0.08 ** | 3.76 | |
| 10 | 1.10 ± 0.12 * | 2.04 | 10 | 0.58 ± 0.19 ** | 5.72 | |
| MDA-MB-436 | 0 | 2.27 ± 0.05 | 0 | 2.36 ± 0.19 | ||
| 0.1 | 1.30 ± 0.05 *** | 1.70 | 0.1 | 1.17 ± 0.12 * | 1.85 | |
| 1 | 0.85 ± 0.06 *** | 2.60 | 1 | 0.77 ± 0.07 ** | 2.78 | |
| 10 | 1.01 ± 0.06 *** | 2.20 | 10 | 0.88 ± 0.38 * | 2.45 | |
| 72 h | A-1 (µM) | Pac IC50 (nM) | Fold decrease compared to Pac Alone | Pac (nM) | A-1 IC50 (µM) | Fold decrease compared to A-1 Alone |
| MDA-MB-231 | 0 | 2.25 ± 0.19 | 0 | 3.33 ± 0.56 | ||
| 0.1 | 1.30 ± 0.05 * | 1.73 | 0.1 | 2.34 ± 0.48 | 1.42 | |
| 1 | 1.08 ± 0.13 * | 2.09 | 1 | 1.03 ± 0.01 * | 3.22 | |
| 10 | 0.70 ± 0.12 ** | 3.22 | 10 | 0.84 ± 0.07 * | 3.93 | |
| MDA-MB-436 | 0 | 2.22 ± 0.12 | 0 | 2.15 ± 0.09 | ||
| 0.1 | 1.48 ± 0.18 | 1.50 | 0.1 | 1.71 ± 0.22 | 1.26 | |
| 1 | 0.10 ± 0.04 ** | 2.22 | 1 | 0.87 ± 0.12 ** | 2.47 | |
| 10 | 0.81 ± 0.20 ** | 2.73 | 10 | 0.72 ± 0.14 ** | 2.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadhosseinpour, S.; Weaver, A.; Sudhakaran, M.; Ho, L.-C.; Le, T.; Doseff, A.I.; Medina-Bolivar, F. Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells. Cancers 2023, 15, 399. https://doi.org/10.3390/cancers15020399
Mohammadhosseinpour S, Weaver A, Sudhakaran M, Ho L-C, Le T, Doseff AI, Medina-Bolivar F. Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells. Cancers. 2023; 15(2):399. https://doi.org/10.3390/cancers15020399
Chicago/Turabian StyleMohammadhosseinpour, Sepideh, Alexx Weaver, Meenakshi Sudhakaran, Linh-Chi Ho, Tra Le, Andrea I. Doseff, and Fabricio Medina-Bolivar. 2023. "Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells" Cancers 15, no. 2: 399. https://doi.org/10.3390/cancers15020399
APA StyleMohammadhosseinpour, S., Weaver, A., Sudhakaran, M., Ho, L. -C., Le, T., Doseff, A. I., & Medina-Bolivar, F. (2023). Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells. Cancers, 15(2), 399. https://doi.org/10.3390/cancers15020399