Influencing Factors on Intersegmental Identification Adequacy in Segmentectomy with Intraoperative Indocyanine Green (ICG) Intravenous Administration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
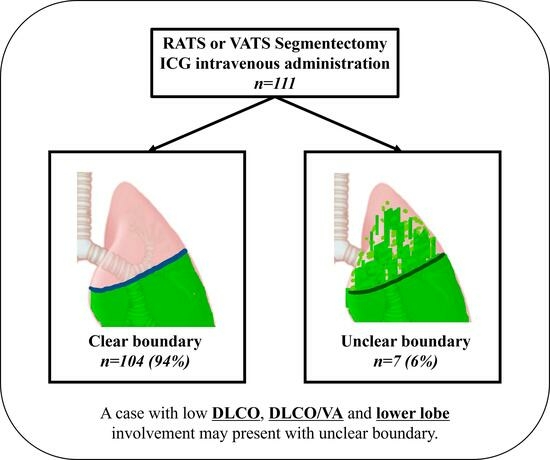
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen-Yoshikawa, T.F.; Fukui, T.; Nakamura, S.; Ito, T.; Kadomatsu, Y.; Tsubouchi, H.; Ueno, H.; Sugiyama, T.; Goto, M.; Mori, S.; et al. Current trends in thoracic surgery. Nagoya J. Med. Sci. 2020, 82, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Aokage, K.; Suzuki, K.; Saji, H.; Wakabayashi, M.; Kataoka, T.; Sekino, Y.; Fukuda, H.; Endo, M.; Hattori, A.; Mimae, T.; et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): A multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir. Med. 2023, 11, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.-H.; Ong, B.-H.; Chao, Y.K.; Wright, G.M.; Sekine, Y.; Wong, I.; Hao, Z.; Zhang, G.; Chaturvedi, H.; Thammineedi, S.R.; et al. Use of Indocyanine Green Fluorescence Imaging in Thoracic and Esophageal Surgery. Ann. Thorac. Surg. 2023, 115, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Goto, M.; Chen-Yoshikawa, T.F. Fluorescence-guided thoracic surgery. J. Vis. Surg. 2021, 7, 18. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F.; Date, H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J. Thorac. Dis. 2016, 8 (Suppl. 3), S295–S301. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Suzuki, J.; Miyoshi, T.; Tane, K.; Samejima, J.; Aokage, K.; Tsuboi, M. Comparison of various lung intersegmental plane identification methods. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 90–97. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Jin, R.; Li, H. Intraoperative Identification of the Intersegmental Plane: From the Beginning to the Future. Thorac. Cancer 2022, 9, 948878. [Google Scholar] [CrossRef]
- Krause, K.; Schumacher, L.Y.; Sachdeva, U.M. Advances in Imaging to Aid Segmentectomy for Lung Cancer. Surg. Oncol. Clin. N. Am. 2022, 31, 595–608. [Google Scholar] [CrossRef]
- Géczi, T.; Simonka, Z.; Lantos, J.; Wetzel, M.; Szabó, Z.; Lázár, G.; Furák, J. Near-infrared fluorescence guided surgery: State of the evidence from a health technology assessment perspective. Front. Surg. 2022, 9, 919739. [Google Scholar] [CrossRef]
- Matsuura, Y.; Ichinose, J.; Nakao, M.; Okumura, S.; Mun, M. Recent fluorescence imaging technology applications of indocyanine green in general thoracic surgery. Surg. Today 2020, 50, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, M.; Potenza, R.; Seguin-Givelet, A.; Gossot, D. Identification of the intersegmental plane during thoracoscopic segmentectomy: State of the art. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Xiaoyan, C.; Linyou, Z. Robot-assisted segmentectomy with improved modified inflation–deflation combined with the intravenous indocyanine green method. J. Robot. Surg. 2023, 17, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Yokomise, H.; Yokota, N.; Yoshida, C.; Katoh, A.; Misaki, N.; Tetsuhiko, G. Dual Image Navigation to Secure Surgical Margins in Thoracoscopic Segmentectomy. Ann. Surg. Oncol. 2023, 30, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Q.; Wang, Z.; Shao, F. Real-time image-guided indocyanine green fluorescence dual-visualization technique to measure the intraoperative resection margin during thoracoscopic segmentectomy. Asia-Pac. J. Clin. Oncol. 2022, 19, E39–E44. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, H.; Ma, L.; Fan, K.; Feng, J.; Zhao, R.; Wen, X.; Zhang, J.; Wu, Q.; Fu, J.; et al. Identification of the intersegmental plane by arterial ligation method during thoracoscopic segmentectomy. J. Cardiothorac. Surg. 2022, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, M.; Okubo, Y.; Yoshida, Y.; Nakagawa, K.; Watanabe, S.-I. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech. 2021, 6, 151–158. [Google Scholar] [CrossRef]
- Matsui, T.; Takahashi, Y.; Nakada, T.; Matsushita, H.; Oya, Y.; Sakakura, N.; Kuroda, H. Efficacy of Xenon Light with Indocyanine Green for Intersegmental Visibility in Thoracoscopic Segmentectomy. J. Surg. Res. 2021, 259, 39–46. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R.; Cao, H. Near-infrared intraoperative imaging with indocyanine green is beneficial in video-assisted thoracoscopic segmentectomy for patients with chronic lung diseases: A retrospective single-center propensity-score matched analysis. Surg. Innov. 2020, 15, 303. [Google Scholar] [CrossRef]
- Kim, Y.; Rho, J.; Quan, Y.H.; Choi, B.H.; Han, K.N.; Kim, H.K.; Choi, Y.H. Simultaneous visualization of pulmonary nodules and intersegmental planes on fluorescent images in pulmonary segmentectomy. Eur. J. Cardiothorac. Surg. 2020, 58 (Suppl. S1), i77–i84. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Q.; Wang, Z.; Shao, F.; Yang, R. Is the near-infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation-deflation method in lung segmentectomy? Thorac. Cancer 2019, 10, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Motono, N.; Iwai, S.; Funasaki, A.; Sekimura, A.; Usuda, K.; Uramoto, H. Low-dose indocyanine green fluorescence-navigated segmentectomy: Prospective analysis of 20 cases and review of previous reports. J. Thorac. Dis. 2019, 11, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Mun, M.; Ichinose, J.; Nakao, M.; Nakagawa, K.; Okumura, S. Recent fluorescence-based optical imaging for video-assisted thoracoscopic surgery segmentectomy. Ann. Transl. Med. 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Pischik, V.G.; Kovalenko, A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J. Thorac. Dis. 2018, 10 (Suppl. S31), S3704–S3711. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.; Okumura, S.; Nakao, M.; Matsuura, Y.; Nakagawa, K. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J. Vis. Surg. 2017, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Guigard, S.; Triponez, F.; Bédat, B.; Vidal-Fortuny, J.; Licker, M.; Karenovics, W. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, S.; Kuroda, H.; Yoshimura, K.; Dejima, H.; Seto, K.; Naomi, A.; Mizuno, T.; Sakakura, N.; Sakao, Y. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J. Thorac. Dis. 2016, 8, 985–991. [Google Scholar] [CrossRef]
- Tarumi, S.; Misaki, N.; Kasai, Y.; Chang, S.S.; Go, T.; Yokomise, H. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur. J. Cardio-Thorac. Surg. 2013, 46, 112–115. [Google Scholar] [CrossRef]
- Kasai, Y.; Tarumi, S.; Chang, S.S.; Misaki, N.; Gotoh, M.; Go, T.; Yokomise, H. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: Comparative analysis of 2- and 1-wavelength methods. Eur. J. Cardio-Thorac. Surg. 2014, 44, 1103–1107. [Google Scholar] [CrossRef]
- Misaki, N.; Tatakawa, K.; Chang, S.S.; Go, T.; Yokomise, H. Constant-rate intravenous infusion of indocyanine green leading to high fluorescence intensity in infrared thoracoscopic segmntectomy. JTCVS Tech. 2020, 3, 319–324. [Google Scholar] [CrossRef]





| Characteristic | Value |
|---|---|
| Clear boundary group/Unclear boundary group | 104 (94)/7 (6) |
| Median Age (Range) | 70 [22–91] |
| Sex, male (%) | 61 (55) |
| Body Mass Index (Median [Range]) | 22.9 [15.0–30.8] |
| CCI (Median [Range]) | 1 [0–9] |
| Smoking Habit, N (%) | 66 (60) |
| FVC, mL (Median [range]) | 3280 [1670–5120] |
| %FVC, % (Median [range]) | 106.5 [71.9–138.1] |
| FEV1, mL (Median [range]) | 2370 [1040–4200] |
| %FEV1,0% (Median [Range]) | 100.4 [44.4–137.5] |
| DLCO, mL/min/mmHg (Median [Range]) | 15.6 [6.4–26.9] |
| DLCO/VA, mL/min/mmHg/L (Median [Range]) | 4.2 [1.4–17.4] |
| Pattern of Ventilatory Impairment, n (%) Within normal limits Restrictive Obstructive Mixed | 73 (66) 1 (1) 36 (32) 1 (1) |
| Clinical Maximum Tumor Size, mm (Median [Range]) | 17 [4–38] |
| Surgical Approach: RATS (%) | 53 (48) |
| Lung Cancer (%) | 85 (77) |
| Segmentectomy Type: Simple (%) | 88 (79) |
| ICG Dose, mg (Median [Range]) | 7.5 [5–20] |
| Median Blood Loss, mL (Range) | 5 [0–200] |
| Operation Duration, Minutes (Median [Range]) | 155 [77–360] |
| Postoperative Complications Grade > 3, n (%) | 9 (8) |
| Postoperative Drainage Duration, Day (Median [Range]) | 2 [1–18] |
| Median Postoperative Hospital Stay (Days) [Range] | 5 [3–28] |
| Characteristic | Clear Boundary Group n = 104 | Unclear Boundary Group (n = 7) | p-Value |
|---|---|---|---|
| Median Age (Range) | 69.5 [22–85] | 75 [47–91] | 0.07 |
| Percentage of Males (%) | 56 (54) | 5 (71) | 0.46 |
| Body Mass Index (Median [Range]) | 23.0 [15–31] | 22 [17–24] | 0.19 |
| CCI (Median [Range]) | 1 [0–9] | 1 [0–8] | 0.97 |
| Brinkman Index (Median [Range]) | 270 [0–2925] | 360 [0–900] | 0.84 |
| VC, mL (Median [Range]) | 3150 [1670–5120] | 3450 [2430–4420] | 0.77 |
| %VC, % (Median [Range]) | 106.0 [72.5–135.4] | 109.9 [71.9–138.1] | 0.21 |
| FEV1, mL (Median [range]) | 2370 [1040–4200] | 2450 [1610–3040] | 0.64 |
| %FEV1,0% (Median [Range]) | 100.2 [44.4–137.5] | 110.7 [64.9–131.5] | 0.26 |
| DLCO, mL/min/mmHg (Median [Range]) | 15.7 [6.4–26.9] | 11.8 [8.1–18.3] | 0.03 |
| DLCO/VA, mL/min/mmHg/L (Median [Range]) | 4.3 [1.4–17.4] | 3.0 [1.7–4.3] | 0.01 |
| Pattern of Ventilatory Impairment (%) Within normal limits Restrictive Obstructive Mixed | 68 (65) 0 35 (34) 1 (1) | 5 (72) 1 (14) 1 (14) 0 | 0.08 |
| Clinical Maximum Tumor Size, mm (Median [Range]) | 17 [4–38] | 23 [14–37] | 0.06 |
| Surgical Approach: RATS (%) | 50 (48) | 3 (43) | 1.00 |
| Lung Cancer (%) | 80 (77) | 5 (71) | 0.67 |
| Segmentectomy Type: Simple (%) | 82 (79) | 6 (86) | 1.00 |
| ICG Dose, mg (Median [Range]) | 7.5 [5–20] | 7.5 [5–10] | 0.89 |
| Median Blood Loss, mL (Range) | 5 [0–105] | 15 [0–200] | 0.13 |
| Operation Duration, Minutes (Median [Range]) | 155 [77–360] | 199 [135–273] | 0.08 |
| Postoperative Complications Grade > 3, n (%) | 8 (8) | 1 (14) | 0.46 |
| Postoperative Drainage Duration, Day (Median [Range]) | 2 [1–18] | 3.5 [2–10] | 0.12 |
| Median Postoperative Hospital Stay (Days) [Range] | 5 [3–28] | 6 [5–26] | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.; Setogawa, T.; Makita, A.; Ohara, Y.; Imamura, Y.; Okado, S.; Watanabe, H.; Kawasumi, Y.; Kadomatsu, Y.; Kato, T.; et al. Influencing Factors on Intersegmental Identification Adequacy in Segmentectomy with Intraoperative Indocyanine Green (ICG) Intravenous Administration. Cancers 2023, 15, 5876. https://doi.org/10.3390/cancers15245876
Ueno H, Setogawa T, Makita A, Ohara Y, Imamura Y, Okado S, Watanabe H, Kawasumi Y, Kadomatsu Y, Kato T, et al. Influencing Factors on Intersegmental Identification Adequacy in Segmentectomy with Intraoperative Indocyanine Green (ICG) Intravenous Administration. Cancers. 2023; 15(24):5876. https://doi.org/10.3390/cancers15245876
Chicago/Turabian StyleUeno, Harushi, Tomohiro Setogawa, Ayaka Makita, Yuko Ohara, Yoshito Imamura, Shoji Okado, Hiroki Watanabe, Yuta Kawasumi, Yuka Kadomatsu, Taketo Kato, and et al. 2023. "Influencing Factors on Intersegmental Identification Adequacy in Segmentectomy with Intraoperative Indocyanine Green (ICG) Intravenous Administration" Cancers 15, no. 24: 5876. https://doi.org/10.3390/cancers15245876
APA StyleUeno, H., Setogawa, T., Makita, A., Ohara, Y., Imamura, Y., Okado, S., Watanabe, H., Kawasumi, Y., Kadomatsu, Y., Kato, T., Nakamura, S., Mizuno, T., & Chen-Yoshikawa, T. F. (2023). Influencing Factors on Intersegmental Identification Adequacy in Segmentectomy with Intraoperative Indocyanine Green (ICG) Intravenous Administration. Cancers, 15(24), 5876. https://doi.org/10.3390/cancers15245876