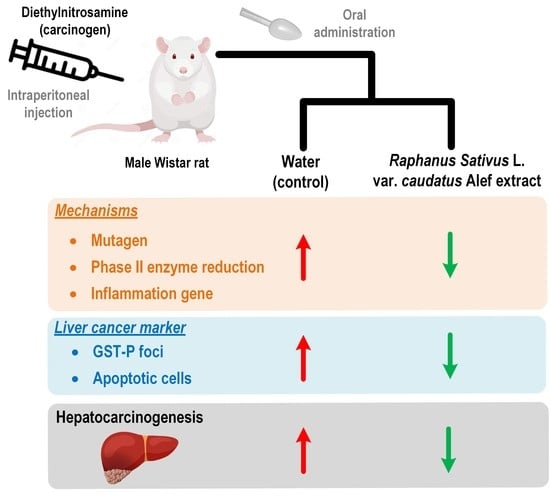
Thai Rat-Tailed Radish Prevents Hepatocarcinogenesis in Rats by Blocking Mutagenicity, Inducing Hepatic Phase II Enzyme, and Decreasing Hepatic Pro-Inflammatory Cytokine Gene Expression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Phytochemical Analysis by HPLC
2.4. Animals and Exposures
2.5. Acute Toxicity Test
2.6. Experimental Design
2.7. Determination of GST-P Positive Foci
2.8. Determination of Apoptotic Cells by TUNEL Assay
2.9. In vitro Mutagenicity and Antimutagenicity Assay
2.10. Determination of Phase II Xenobiotic-Metabolizing Enzymes
2.11. Determination of Pro-Inflammatory Cytokine Gene Expression by Real-Time PCR
2.12. Statistical Analysis
3. Results
3.1. Phytochemical Identification
3.2. Toxicity of RS
3.3. Effect of RS on GST-P Positive Foci
3.4. Effect of RS on Apoptosis Induction
3.5. In Vitro Mutagenic and Antimutagenic Activity of RS
3.6. Effect of RS on Phase II Xenobiotic-Metabolizing Enzymes
3.7. Effect of RS on Pro-Inflammatory Cytokine Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanlier, N.; Saban, G. The benefits of brassica vegetables on human health. J. Hum. Health Res. 2018, 1, 1–13. [Google Scholar]
- Peluso, M.; Munnia, A.; Russo, V.; Galli, A.; Pala, V.; Schouw, Y.T.V.D.; Schulze, M.B.; Weiderpass, E.; Tumino, R.; Saieva, C.; et al. Cruciferous vegetable intake and bulky DNA damage within non-smokers and former smokers in the Gen-Air study (EPIC Cohort). Nutrients 2022, 14, 2477. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ngai, C.H.; Deng, Y.; Tin, M.S.; Lok, V.; Zhang, L.; Yuan, J.; Xu, W.; Zheng, Z.-J.; Wong, M.C.S. Cancer incidence and mortality in Asian countries: A trend analysis. Cancer Control 2022, 29, 10732748221095955. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocasap, P.; Weerapreeyakul, N.; Barusrux, S. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef). J. Funct. Foods 2013, 5, 1372–1381. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N.; Lehtonen, M.; Leppanen, J.; Rautio, J. High-accuracy mass spectrometry for identification of sulphur-containing bioactive constituents and flavonoids in extracts of Raphanus sativus var. caudatus Alef (Thai rat-tailed radish). J. Funct. Foods 2017, 31, 237–247. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Thumanu, K. Structures of isothiocyanates attributed to reactive oxygen species generation and microtubule depolymerization in HepG2 cells. Biomed. Pharmacother. 2018, 101, 698–709. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N. Simultaneous quantification of sulforaphene and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016, 201, 139–144. [Google Scholar] [CrossRef]
- Yongpradoem, P.; Weerapreeyakul, N. Evaluation of antioxidant activity and inhibition of tyrosinase activity of Raphanus sativus var. caudatus Alef extract. Walailak J. Sci. Technol. 2020, 17, 838–850. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Sankam, P.; Wongpoomchai, R. Inhibitory effect of Thai purple rice husk extract on chemically induced carcinogenesis in rats. Molecules 2021, 26, 360. [Google Scholar] [CrossRef]
- Insuan, O.; Charoensin, S.; Roytrakul, S.; Thumvijit, T.; Bunpo, P.; Wongpoomchai, R. Carcinogenicity and proteomic analysis of N-nitrosodiethylamine in rats. Vet. Integr. Sci. 2018, 16, 109–122. [Google Scholar]
- Khuanphram, N.; Taya, S.; Kongtawelert, P.; Wongpoomchai, R. Sesame extract promotes chemopreventive effect of hesperidin on early phase of diethylnitrosamine-initiated hepatocarcinogenesis in rats. Pharmaceutics 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Kittichaiworakul, R.; Taya, S.; Chariyakornkul, A.; Chaiyaso, T.; Wongpoomchai, R. Antigenotoxic effects and possible mechanism of red yeast (Sporidiobolus pararoseus) on Aflatoxin B1-induced mutagenesis. Biomolecules 2021, 11, 734. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [Green Version]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Zhang, N.; Jing, P. Anthocyanins in Brassicaceae: Composition, stability, bioavailability, and potential health benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 2205–2220. [Google Scholar] [CrossRef] [PubMed]
- Al Hilfi, Z.A.F.; Nencu, I.; Costea, T.; Gird, C.E.; Stoicescu, C.S.; Anghel, A.I.; Negres, S.J.F. Chemical composition and antioxidant activity of Ficus elastica Roxb. ex Hornem and Raphanus sativus L. selective dry extracts with potential antidiabetic activity. Farmasia 2019, 67, 5. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Baskar, T.B.; Park, S.U. Total phenolic and flavonoid contents and antioxidant activities of two Raphanus sativus L. cultivars (cherry belle and valentine). Biosci. Biotechnol. Res. Asia 2016, 13, 31–36. [Google Scholar] [CrossRef]
- Agarwal, K.; Varma, R.J. Radical scavenging ability and biochemical screening of a common Asian vegetable-Raphanus sativus L. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 127–134. [Google Scholar]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Kim, B.; Kim, S.-H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat. Res.-Fundam. Mech. Mutagen. 2004, 555, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Pocasap, P.; Weerapreeyakul, N.; Tanthanuch, W.; Thumanu, K. Sulforaphene in Raphanus sativus L. var. caudatus Alef increased in late-bolting stage as well as anticancer activity. Asian Pac. J. Trop. Biomed. 2017, 7, 998–1004. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Thumanu, K. Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomol. Ther. 2019, 27, 540–552. [Google Scholar] [CrossRef]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015, 49, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Muriel, P.; Ramos-Tovar, E.; Montes-Páez, G.; Buendía-Montaño, L.D. Chapter 40—Experimental models of liver damage mediated by oxidative stress. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 529–546. [Google Scholar]
- Noguti, J.; Barbisan, L.F.; Cesar, A.; Dias Seabra, C.; Choueri, R.B.; Ribeiro, D.A. Review: In vivo models for measuring placental glutatione-S-transferase (GST-P 7-7) levels: A suitable biomarker for understanding cancer pathogenesis. In Vivo 2012, 26, 647–650. [Google Scholar] [PubMed]
- Pocasap, P.; Weerapreeyakul, N.; Wongpoomchai, R. Chemopreventive effect of Cratoxylum formosum (Jack) ssp. pruniflorum on initial stage hepatocarcinogenesis in rats. Molecules 2021, 26, 4235. [Google Scholar] [CrossRef]
- Gründemann, C.; Huber, R. Chemoprevention with isothiocyanates—From bench to bedside. Cancer Lett. 2018, 414, 26–33. [Google Scholar] [CrossRef]
- Shishu; Kaur, I.P. Inhibition of cooked food-induced mutagenesis by dietary constituents: Comparison of two natural isothiocyanates. Food Chem. 2009, 112, 977–981. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iwahashi, T.; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish). J. Agric. Food Chem. 2001, 49, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Przychodzeń, W.; Kusznierewicz, B.; Kołodziejski, D.; Namieśnik, J.; Bartoszek, A. Isothiocyanates may chemically detoxify mutagenic amines formed in heat processed meat. Food Chem. 2014, 157, 105–110. [Google Scholar] [CrossRef]
- Nilnumkhum, A.; Punvittayagul, C.; Chariyakornkul, A.; Wongpoomchai, R. Effects of hydrophilic compounds in purple rice husk on AFB1-induced mutagenesis. Mol. Cell. Toxicol. 2017, 13, 171–178. [Google Scholar] [CrossRef]
- Singh, D.; Arora, R.; Bhatia, A.; Singh, H.; Singh, B.; Arora, S. Molecular targets in cancer prevention by 4-(methylthio)butyl isothiocyanate—A comprehensive review. Life Sci. 2020, 241, 117061. [Google Scholar] [CrossRef] [PubMed]
- Velli, S.K.; Sundaram, J.; Murugan, M.; Balaraman, G.; Thiruvengadam, D. Protective effect of vanillic acid against benzo(a)pyrene induced lung cancer in Swiss albino mice. J. Biochem. Mol. Toxicol. 2019, 33, e22382. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Cheung, K.L.; Kong, A.-N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.A.; Holbrook, J.T.; Criner, G.; Sethi, S.; Rayapudi, S.; Sudini, K.R.; Sugar, E.A.; Burke, A.; Thimmulappa, R.; Singh, A.; et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: A randomized, double-Blind, placebo controlled trial. PLoS ONE 2016, 11, e0163716. [Google Scholar] [CrossRef] [Green Version]
- Stanely Mainzen Prince, P.; Rajakumar, S.; Dhanasekar, K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 668, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.; Ajijola, I.; Agbeti, O. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum. Exp. Toxicol. 2019, 38, 1254–1265. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A.; Singh, H.; Kaur, S.; Arora, S.; Singh, B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-κB, TNF-α and COX-2 proteins in rats. Phytother. Res. 2022, 36, 1338–1352. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Vinayagam, R.; Arokia Vijaya Anand, M.; Isa, N.M.; Ponnaiyan, R. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: A review. Toxicol. Res. 2020, 9, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Rinaldi, L.; Giordano, M.; Marrone, A.; Adinolfi, L.E. Mechanisms and clinical behavior of hepatocellular carcinoma in HBV and HCV infection and alcoholic and non-alcoholic fatty liver disease. Hepatoma Res. 2018, 4, 55. [Google Scholar] [CrossRef]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin use decreases the incidence of hepatocellular carcinoma: An updated meta-analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef] [Green Version]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Bandoh, S.; Roberts, L.R. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Research 2016, 5, 879. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Gene | Primer Sequence | Reference | |
|---|---|---|---|
| Nrf-2 | Forward | 5′-GCC AGC TGA ACT CCT TAG AC-3′ | [10] |
| Reverse | 5′-GAT TCG TGC ACA GCA GCA-3′ | ||
| Tnf-α | Forward | 5′-AAA TGG CCC TCT CAT CAG TCC-3′ | [15] |
| Reverse | 5′-TCT GCT TGG TGG TTT GCT ACG AC-3′ | ||
| β-actin | Forward | 5′-ACA GGA TGC AGA AGG AGA TTA C-3′ | [15] |
| Reverse | 5′-AGA GTG AGG CCA GGA TAG A-3′ |
| Phytochemicals | Extract (mg/g Extract) | ||
|---|---|---|---|
| RS-H2O | RS-DCM | ||
| Phenolics | |||
| Protocatechuic acid | 4.21 ± 1.60 | 2.33 ± 0.03 | |
| p-Hydroxybenzoic acid | 1.06 ± 0.18 | 6.01 ± 0.06 | |
| Vanillic acid | 26.15 ± 8.97 | nd | |
| Caffeic acid | 0.63 ± 0.05 | 0.43 ± 0.02 | |
| p-Coumaric acid | 0.66 ± 0.06 | nd | |
| Ferulic acid | nd | 2.20 ± 0.01 | |
| Isothiocyanates | |||
| Sulforaphane | nd | nd | |
| Sulforaphene | 0.72 ± 0.02 | 5.11 ± 0.23 | |
| Treatments | Weight (g) | Consumption (g/rat/day) | ||
|---|---|---|---|---|
| Initial | Final | Food | Water | |
| DW | 172 ± 12 | 181 ± 14 | 13 ± 1 | 25 ± 0 |
| RS-H2O (5000 mg/kg BW) | 173 ± 16 | 205 ± 14 | 14 ± 0 | 29 ± 3 |
| Organs | Treatments | ||
|---|---|---|---|
| DW | RS-H2O | ||
| (5000 mg/kg BW) | |||
| Relative weight (g/100 mg BW) | Liver | 3.19 ± 0.15 | 4.21 ± 0.45 |
| Spleen | 0.20 ± 0.02 | 0.20 ± 0.03 | |
| Kidney | 0.76 ± 0.05 | 0.85 ± 0.05 | |
| Lung | 0.45 ± 0.31 | 0.43 ± 0.01 | |
| Heart | 0.31 ± 0.03 | 0.30 ± 0.02 | |
| Pancreas | 0.38 ± 0.06 | 0.38 ± 0.06 | |
| Adrenal gland | 0.0366 ± 0.0055 | 0.0402 ± 0.0056 | |
| Ovary | 0.0631 ± 0.0118 | 0.0560 ± 0.0097 | |
| Uterus | 0.27 ± 0.08 | 0.25 ± 0.06 | |
| Group | Treatment | Weight (g) | Consumption (g/rat/day) | |||
|---|---|---|---|---|---|---|
| Initiator # | Test Compound | Initial | Final | Food | Water | |
| 1 | DEN | DW | 88 ± 17 | 271 ± 17 | 21 ± 0 | 27 ± 9 |
| 2 | DEN | RS-H2O 500 mg/kg | 86 ± 18 | 260 ± 22 | 21 ± 0 | 31 ± 10 |
| 3 | DEN | RS-H2O 100 mg/kg | 83 ± 16 | 261 ± 21 | 21 ± 1 | 27 ± 5 |
| 4 | DEN | RS-DCM 20 mg/kg | 86 ± 18 | 271 ± 19 | 21 ± 2 | 23 ± 7 |
| 5 | NSS | DW | 80 ± 14 | 289 ± 20 | 21 ± 0 | 31 ± 8 |
| 6 | NSS | RS-H2O 500 mg/kg | 89 ± 22 | 300 ± 19 | 23 ± 2 | 27 ± 1 |
| 7 | NSS | RS-H2O 100 mg/kg | 86 ± 17 | 308 ± 24 | 21 ± 1 | 30 ± 7 |
| 8 | NSS | RS-DCM 20 mg/kg | 84 ± 20 | 297 ± 31 | 20 ± 2 | 25 ± 8 |
| Treatments | TA98 | TA100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | ||||||
| Dose (mg/plate) | Revertant Colonies | MI | Revertant Colonies | MI | Revertant Colonies | MI | Revertant Colonies | MI | |
| Negative control | |||||||||
| DMSO | 17.1 ± 3.2 | 23.4 ± 4.9 | 109.4 ± 15.9 | 95.0 ± 12.6 | |||||
| DW | 24.0 ± 3.8 | 29.4 ± 5.1 | 106.4 ± 14.6 | 99.0 ± 15.9 | |||||
| Positive control | |||||||||
| AF-2 | 1 × 10−4 | 388.8 ± 38.5 * | |||||||
| 1 × 10−5 | 427.2 ± 39.9 * | ||||||||
| 2-AA | 5 × 10−4 | 618.0 ± 52.1 * | 1452.0 ± 144.7 * | ||||||
| RS-H2O | 0.1 | 18.8 ± 2.5 | 0.8 | 25.9 ± 5.4 | 0.9 | 96.2 ± 17.9 | 0.9 | 97.3 ± 15.8 | 1.0 |
| 0.5 | 21.3 ± 5.6 | 0.9 | 27.8 ± 3.9 | 0.9 | 101.5 ± 15.5 | 1.0 | 111.2 ± 13.8 | 1.1 | |
| 1.0 | 19.6 ± 2.8 | 0.8 | 29.8 ± 5.2 | 1.0 | 99.7 ± 21.8 | 0.9 | 109.6 ± 12.8 | 1.1 | |
| 5.0 | 19.0 ± 4.5 | 0.8 | 34.1 ± 7.4 | 1.2 | 117.1 ± 13.7 | 1.1 | 115.3 ± 17.6 | 1.2 | |
| RS-DCM | 0.1 | 18.8 ± 3.2 | 1.1 | 23.9 ± 4.8 | 1.0 | 106.1 ± 13.1 | 1.0 | 93.2 ± 18.7 | 1.0 |
| 0.5 | 19.4 ± 8.0 | 1.1 | 24.8 ± 2.0 | 1.1 | 79.3 ± 16.5 | 0.7 | 88.6 ± 13.8 | 0.9 | |
| 1.0 | 16.6 ± 5.9 | 1.0 | 22.1 ± 3.7 | 0.9 | 66.2 ± 23.3 | 0.6 | 91.7 ± 13.4 | 1.0 | |
| 5.0 | 5.6 ± 5.9 * | 0.3 K | 18.1 ± 6.2 | 0.8 | 40.3 ± 33.0 * | 0.4 K | 94.5 ± 21.6 | 1.0 | |
| Treatment | Revertant Colonies | ||||
|---|---|---|---|---|---|
| Dose | TA98 | TA100 | |||
| (mg/plate) | −S9 | +S9 | −S9 | +S9 | |
| Negative control | |||||
| DMSO | 18.7 ± 4.5 * | 21.7 ± 3.1 * | 91.2 ± 6.9 * | 85.3 ± 14.2 * | |
| DW | 20.2 ± 4.1 * | 32.3 ± 3.2 * | 98.6 ± 2.4 * | 97.7 ± 16.0 * | |
| Positive control | |||||
| AF-2 | 1 × 10−4 | 291.5 ± 31.4 | |||
| AFB1 | 1 × 10−5 | 1233.3 ± 35.3 | |||
| NaN3 | 5 × 10−4 | 370.8 ± 44.9 | |||
| MeIQ | 5 × 10−4 | 1056.3 ± 65.6 | |||
| RS-H2O | 0.1 | 393.2 ± 14.4 * | 1198.7 ± 70.7 * | 412.0 ± 43.7 | 836.7 ± 137.1 * |
| 0.5 | 374.8 ± 25.2 * | 1079.7 ± 62.4 | 387.7 ± 34.8 | 1038.8 ± 170.0 | |
| 1.0 | 389.2 ± 26.8 * | 1128.0 ± 6.5 * | 349.2 ± 48.7 | 1317.3 ± 125.9 * | |
| RS-DCM | 0.1 | 222.0 ± 20.3 * | 762.7 ± 6.1 * | 354.7 ± 53.7 | 997.8 ± 116.0 |
| 0.5 | 123.0 ± 19.3 * | 127.3 ± 4.0 * | 164.5 ± 26.3 * | 606.7 ± 80.1 * | |
| 1.0 | 57.7 ± 30.6 * | 24.3 ± 6.5 * | 98.0 ± 14.0 * | 296.0 ± 65.4 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocasap, P.; Weerapreeyakul, N.; Wongpoomchai, R. Thai Rat-Tailed Radish Prevents Hepatocarcinogenesis in Rats by Blocking Mutagenicity, Inducing Hepatic Phase II Enzyme, and Decreasing Hepatic Pro-Inflammatory Cytokine Gene Expression. Cancers 2023, 15, 1906. https://doi.org/10.3390/cancers15061906
Pocasap P, Weerapreeyakul N, Wongpoomchai R. Thai Rat-Tailed Radish Prevents Hepatocarcinogenesis in Rats by Blocking Mutagenicity, Inducing Hepatic Phase II Enzyme, and Decreasing Hepatic Pro-Inflammatory Cytokine Gene Expression. Cancers. 2023; 15(6):1906. https://doi.org/10.3390/cancers15061906
Chicago/Turabian StylePocasap, Piman, Natthida Weerapreeyakul, and Rawiwan Wongpoomchai. 2023. "Thai Rat-Tailed Radish Prevents Hepatocarcinogenesis in Rats by Blocking Mutagenicity, Inducing Hepatic Phase II Enzyme, and Decreasing Hepatic Pro-Inflammatory Cytokine Gene Expression" Cancers 15, no. 6: 1906. https://doi.org/10.3390/cancers15061906
APA StylePocasap, P., Weerapreeyakul, N., & Wongpoomchai, R. (2023). Thai Rat-Tailed Radish Prevents Hepatocarcinogenesis in Rats by Blocking Mutagenicity, Inducing Hepatic Phase II Enzyme, and Decreasing Hepatic Pro-Inflammatory Cytokine Gene Expression. Cancers, 15(6), 1906. https://doi.org/10.3390/cancers15061906