Imaging GRPr Expression in Metastatic Castration-Resistant Prostate Cancer with [68Ga]Ga-RM2—A Head-to-Head Pilot Comparison with [68Ga]Ga-PSMA-11
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
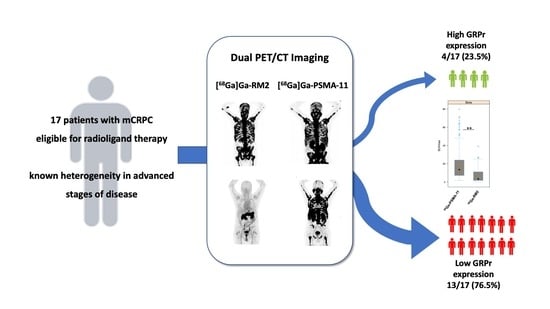
2.1. Patient Population
2.2. Radiotracer Preparation
2.3. PET/CT Imaging and Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Uptake Comparison between [68Ga]Ga-PSMA-11 and [68Ga]Ga-RM2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 2 August 2022).
- Mateo, J.; McKay, R.; Abida, W.; Aggarwal, R.; Alumkal, J.; Alva, A.; Feng, F.; Gao, X.; Graff, J.; Hussain, M.; et al. Accelerating precision medicine in metastatic prostate cancer. Nat. Cancer 2020, 1, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Schmidt, M.; Heinzel, A.; Eppard, E.; Bode, A.; Yordanova, A.; Claesener, M.; Ahmadzadehfar, H. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J. Nucl. Med. 2016, 57, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kurpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef]
- Duan, H.; Baratto, L.; Fan, R.E.; Soerensen, S.J.C.; Liang, T.; Chung, B.I.; Thong, A.E.C.; Gill, H.; Kunder, C.; Stoyanova, T.; et al. Correlation of (68)Ga-RM2 PET with Postsurgery Histopathology Findings in Patients with Newly Diagnosed Intermediate- or High-Risk Prostate Cancer. J. Nucl. Med. 2022, 63, 1829–1835. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; de Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [(6)(8)Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 964–973. [Google Scholar] [CrossRef]
- Ananias, H.J.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef]
- Duan, H.; Ghanouni, P.; Daniel, B.; Rosenberg, J.; Thong, A.; Kunder, C.; Mari Aparici, C.; Davidzon, G.A.; Moradi, F.; Sonn, G.A.; et al. A Pilot Study of (68)Ga-PSMA11 and (68)Ga-RM2 PET/MRI for Biopsy Guidance in Patients with Suspected Prostate Cancer. J. Nucl. Med. 2022, 64, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Ghezzo, S.; Samanes Gajate, A.M.; Preza, E.; Brembilla, G.; Cucchiara, V.; Ahmed, N.; Bezzi, C.; Presotto, L.; Bettinardi, V.; et al. Preliminary Results of an Ongoing Prospective Clinical Trial on the Use of (68)Ga-PSMA and (68)Ga-DOTA-RM2 PET/MRI in Staging of High-Risk Prostate Cancer Patients. Diagnostics 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of (6)(8)Ga-RM2 PET and (6)(8)Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Sonni, I.; Hancock, S.; Vasanawala, S.; Loening, A.; Gambhir, S.S.; Iagaru, A. Prospective Evaluation of (68)Ga-RM2 PET/MRI in Patients with Biochemical Recurrence of Prostate Cancer and Negative Findings on Conventional Imaging. J. Nucl. Med. 2018, 59, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ghanouni, P.; Daniel, B.; Rosenberg, J.; Davidzon, G.A.; Aparici, C.M.; Kunder, C.; Sonn, G.A.; Iagaru, A. A Pilot Study of (68)Ga-PSMA11 and (68)Ga-RM2 PET/MRI for Evaluation of Prostate Cancer Response to High-Intensity Focused Ultrasound Therapy. J. Nucl. Med. 2023, 64, 592–597. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Fassbender, T.F.; Schiller, F.; Zamboglou, C.; Drendel, V.; Kiefer, S.; Jilg, C.A.; Grosu, A.L.; Mix, M. Voxel-based comparison of [(68)Ga]Ga-RM2-PET/CT and [(68)Ga]Ga-PSMA-11-PET/CT with histopathology for diagnosis of primary prostate cancer. EJNMMI Res. 2020, 10, 62. [Google Scholar] [CrossRef]
- Iagaru, A. Will GRPR Compete with PSMA as a Target in Prostate Cancer? J. Nucl. Med. 2017, 58, 1883–1884. [Google Scholar] [CrossRef]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kubler, H.; Haberkorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gage, K.L.; Faraj, S.F.; Macura, K.J.; Cornish, T.C.; Gonzalez-Roibon, N.; Guner, G.; Munari, E.; Partin, A.W.; Pavlovich, C.P.; et al. (1)(8)F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J. Nucl. Med. 2015, 56, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.M.; Djannatian, M.; Czech, N.; Nitsche, M. Prostate-Specific Membrane Antigen PET/CT: False-Positive Results due to Sarcoidosis? Case Rep. Oncol. 2016, 9, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Song, H.; Duan, H.; Hatami, N.; Bagshaw, H.P.; Buyyounouski, M.; Hancock, S.; Shah, S.; Srinivas, S.; Swift, P.; et al. PSMA- and GRPR-Targeted PET: Results from 50 Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2021, 62, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Koller, L.; Joksch, M.; Schwarzenbock, S.; Kurth, J.; Heuschkel, M.; Holzleitner, N.; Beck, R.; von Amsberg, G.; Wester, H.J.; Krause, B.J.; et al. Preclinical Comparison of the (64)Cu- and (68)Ga-Labeled GRPR-Targeted Compounds RM2 and AMTG, as Well as First-in-Humans [(68)Ga]Ga-AMTG PET/CT. J. Nucl. Med. 2023, 64, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Kurth, J.; Krause, B.J.; Schwarzenbock, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [(177)Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; Nock, B.A.; et al. 68Ga/177Lu-NeoBOMB1, a Novel Radiolabeled GRPR Antagonist for Theranostic Use in Oncology. J. Nucl. Med. 2017, 58, 293–299. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T.; et al. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef]
- Mansi, R.; Fleischmann, A.; Macke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers--from basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Gruber, L.; Jimenez-Franco, L.D.; Decristoforo, C.; Uprimny, C.; Glatting, G.; Hohenberger, P.; Schoenberg, S.O.; Reindl, W.; Orlandi, F.; Mariani, M.; et al. MITIGATE-NeoBOMB1, a Phase I/IIa Study to Evaluate Safety, Pharmacokinetics, and Preliminary Imaging of (68)Ga-NeoBOMB1, a Gastrin-Releasing Peptide Receptor Antagonist, in GIST Patients. J. Nucl. Med. 2020, 61, 1749–1755. [Google Scholar] [CrossRef]
- Michalski, K.; Kemna, L.; Asberger, J.; Grosu, A.L.; Meyer, P.T.; Ruf, J.; Sprave, T. Gastrin-Releasing Peptide Receptor Antagonist [(68)Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer. Cancers 2021, 13, 6106. [Google Scholar] [CrossRef]
- Colbert, L.E.; Rebueno, N.; Moningi, S.; Beddar, S.; Sawakuchi, G.O.; Herman, J.M.; Koong, A.C.; Das, P.; Holliday, E.B.; Koay, E.J.; et al. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv. Radiat. Oncol. 2018, 3, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, S.; Nakamura, Y.; Maekawa, T.; Akahira, J.; Miki, Y.; Suzuki, T.; Ishidoya, S.; Arai, Y.; Sasano, H. Immunohistochemical analysis of gastrin-releasing peptide receptor (GRPR) and possible regulation by estrogen receptor betacx in human prostate carcinoma. Neoplasma 2012, 59, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Muntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Davidzon, G.A.; Moradi, F.; Liang, T.; Song, H.; Iagaru, A. Modified PROMISE Criteria for Standardized Interpretation of Gastrin Realising Peptide Receptor (GRPR)-targeted PET. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: http://www.R-project.org/ (accessed on 31 March 2022).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Moller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Francis, R.J.; et al. TheraP: 177Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel—Overall survival after median follow-up of 3 years (ANZUP 1603). J. Clin. Oncol. 2022, 40, 5000. [Google Scholar] [CrossRef]
- Merkens, L.; Sailer, V.; Lessel, D.; Janzen, E.; Greimeier, S.; Kirfel, J.; Perner, S.; Pantel, K.; Werner, S.; von Amsberg, G. Aggressive variants of prostate cancer: Underlying mechanisms of neuroendocrine transdifferentiation. J. Exp. Clin. Cancer Res. 2022, 41, 46. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.; Beyaert, R. Inflammation and NF-kappaB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Matouk, I.; Raveh, E.; Ohana, P.; Lail, R.A.; Gershtain, E.; Gilon, M.; De Groot, N.; Czerniak, A.; Hochberg, A. The increasing complexity of the oncofetal h19 gene locus: Functional dissection and therapeutic intervention. Int. J. Mol. Sci. 2013, 14, 4298–4316. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.C.; Fu, W.M.; Wong, C.W.; Wang, Y.; Wang, W.M.; Hu, G.X.; Zhang, L.; Xiao, L.J.; Wan, D.C.; Zhang, J.F.; et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 2015, 6, 22513–22525. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Danza, G.; Di Serio, C.; Rosati, F.; Lonetto, G.; Sturli, N.; Kacer, D.; Pennella, A.; Ventimiglia, G.; Barucci, R.; Piscazzi, A.; et al. Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol. Cancer Res. 2012, 10, 230–238. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- de Visser, M.; van Weerden, W.M.; de Ridder, C.M.; Reneman, S.; Melis, M.; Krenning, E.P.; de Jong, M. Androgen-dependent expression of the gastrin-releasing peptide receptor in human prostate tumor xenografts. J. Nucl. Med. 2007, 48, 88–93. [Google Scholar]
- Yu, Z.; Ananias, H.J.; Carlucci, G.; Hoving, H.D.; Helfrich, W.; Dierckx, R.A.; Wang, F.; de Jong, I.J.; Elsinga, P.H. An update of radiolabeled bombesin analogs for gastrin-releasing peptide receptor targeting. Curr. Pharm. Des. 2013, 19, 3329–3341. [Google Scholar] [CrossRef]
- Constantinides, C.; Lazaris, A.C.; Haritopoulos, K.N.; Pantazopoulos, D.; Chrisofos, M.; Giannopoulos, A. Immunohistochemical detection of gastrin releasing peptide in patients with prostate cancer. World J. Urol. 2003, 21, 183–187. [Google Scholar] [CrossRef]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef]
- Verhoeven, M.; Ruigrok, E.A.M.; van Leenders, G.; van den Brink, L.; Balcioglu, H.E.; van Weerden, W.M.; Dalm, S.U. GRPR versus PSMA: Expression profiles during prostate cancer progression demonstrate the added value of GRPR-targeting theranostic approaches. Front. Oncol. 2023, 13, 1199432. [Google Scholar] [CrossRef]
- Annunziata, S.; Cuccaro, A.; Calcagni, M.L.; Hohaus, S.; Giordano, A.; Rufini, V. Interim FDG-PET/CT in Hodgkin lymphoma: The prognostic role of the ratio between target lesion and liver SUVmax (rPET). Ann. Nucl. Med. 2016, 30, 588–592. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, K.; Hu, S.; Huang, Y.; Ma, L.; Li, M.; Song, Y. The PET-Derived Tumor-to-Liver Standard Uptake Ratio (SUV (TLR) ) Is Superior to Tumor SUVmax in Predicting Tumor Response and Survival After Chemoradiotherapy in Patients With Locally Advanced Esophageal Cancer. Front. Oncol. 2020, 10, 1630. [Google Scholar] [CrossRef]
- Mansi, R.; Wang, X.; Forrer, F.; Waser, B.; Cescato, R.; Graham, K.; Borkowski, S.; Reubi, J.C.; Maecke, H.R. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 97–107. [Google Scholar] [CrossRef]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Hull, W.E.; Wangler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Damle, N.A.; Shamim, S.A.; Kumar, R.; Seth, A.; Bal, C. Long-term outcome of 177Lu-PSMA-617 radioligand therapy in heavily pre-treated metastatic castration-resistant prostate cancer patients. PLoS ONE 2021, 16, e0251375. [Google Scholar] [CrossRef]
- Beheshti, M.; Taimen, P.; Kemppainen, J.; Jambor, I.; Muller, A.; Loidl, W.; Kahkonen, E.; Kakela, M.; Berndt, M.; Stephens, A.W.; et al. Value of (68)Ga-labeled bombesin antagonist (RM2) in the detection of primary prostate cancer comparing with [(18)F]fluoromethylcholine PET-CT and multiparametric MRI-a phase I/II study. Eur. Radiol. 2023, 33, 472–482. [Google Scholar] [CrossRef]
- Fernández, R.; Kramer, V.; Hurtado de Mendoza, A.; Flores, J.; Amaral, H. Preliminary Evaluation of Tumor Uptake and Laboratory parameters After a Single Dose of 177Lu-RM2 Radioligand therapy in Metastatic Castrate-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 952. [Google Scholar] [CrossRef]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O’Brien, J.; et al. Administering [(177)Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur. Urol. 2023. [Google Scholar] [CrossRef] [PubMed]




| Patient No. | Age (y) | Gleason Score | PSA (ng/mL) | Previous | [68Ga]Ga-PSMA-11 * | [68Ga]Ga-RM2 | Delay |
|---|---|---|---|---|---|---|---|
| Treatments | (Days) | ||||||
| 1 | 63 | NA | NA | QT + RT + ARSI | B + P | B + P | 1 |
| 2 | 65 | NA | 1206 | RT + ARSI | B + LN + P + ST | B + LN + P | 2 |
| 3 | 71 | NA | 7.94 | S + QT + RT + ARSI | LN | LN | 14 |
| 4 | 53 | 7 | NA | ARSI | LN + B + P | LN + B + P | 1 |
| 5 | 76 | NA | 88.4 | S + RT + ARSI | B | B | 18 |
| 6 | 54 | 7(4 + 3) | 470 | ARSI | B + LN + P + ST | B + LN + P + ST | 1 |
| 7 | 75 | NA | NA | QT + RT + ARSI | B + P | B + P | 7 |
| 8 | 73 | NA | 660 | QT + RT + ARSI | B + LN + P | B + LN + P | 2 |
| 9 | 53 | 8 | 1 | RT ** | P + LN | P | 6 |
| 10 | 70 | 6 | 7.11 | NA | P + LN | P + LN | 14 |
| 11 | 68 | NA | 40.1 | S + QT + RT + ARSI | B + LN + P | P | 14 |
| 12 | 64 | NA | 1365 | RT + ARSI | B + LN + P + ST | B + LN | 3 |
| 13 | 55 | 8 | 0.05 | S + RT + ARSI | B + LN + ST | B + LN + ST | 18 |
| 14 | 71 | NA | NA | NA | LN + P | P | 6 |
| 15 | 66 | NA | 79.37 | S + ARSI | B | B | 4 |
| 16 | 64 | 4 + 3 | 3.6 | S + RT + ARSI | B | B | 33 |
| 17 | 71 | NA | 18 | QT + RT + ARSI | B | NL | 6 |
| Region | Parameter | [68Ga]Ga-PSMA-11 | [68Ga]Ga-RM2 | p-Value |
|---|---|---|---|---|
| Bone | SUVmax | 17.0 ± 5.2 | 11.0 ± 5.9 | 0.0029 |
| TBR | 15.9 ± 10.9 | 4.3 ± 5.4 | 0.0023 | |
| LN | SUVmax | 15.7 ± 10.7 | 3.5 ±6.0 | 0.028 |
| TBR | 16.0 ± 12.4 | 5.7 ± 5.2 | 0.038 | |
| Prostate | SUVmax | 16.8 ± 12.2 | 4.8 ± 4.2 | 0.002 |
| TBR | 16.5 ± 13.0 | 5.9 ± 4.6 | 0.002 | |
| Soft tissue | SUVmax | 9.8 ± 3.2 | 1.7 ± 2.1 | 0.06 |
| TBR | 13.7 ± 11.4 | 1.5 ± 1.7 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, R.; Soza-Ried, C.; Iagaru, A.; Stephens, A.; Müller, A.; Schieferstein, H.; Sandoval, C.; Amaral, H.; Kramer, V. Imaging GRPr Expression in Metastatic Castration-Resistant Prostate Cancer with [68Ga]Ga-RM2—A Head-to-Head Pilot Comparison with [68Ga]Ga-PSMA-11. Cancers 2024, 16, 173. https://doi.org/10.3390/cancers16010173
Fernández R, Soza-Ried C, Iagaru A, Stephens A, Müller A, Schieferstein H, Sandoval C, Amaral H, Kramer V. Imaging GRPr Expression in Metastatic Castration-Resistant Prostate Cancer with [68Ga]Ga-RM2—A Head-to-Head Pilot Comparison with [68Ga]Ga-PSMA-11. Cancers. 2024; 16(1):173. https://doi.org/10.3390/cancers16010173
Chicago/Turabian StyleFernández, René, Cristian Soza-Ried, Andrei Iagaru, Andrew Stephens, Andre Müller, Hanno Schieferstein, Camilo Sandoval, Horacio Amaral, and Vasko Kramer. 2024. "Imaging GRPr Expression in Metastatic Castration-Resistant Prostate Cancer with [68Ga]Ga-RM2—A Head-to-Head Pilot Comparison with [68Ga]Ga-PSMA-11" Cancers 16, no. 1: 173. https://doi.org/10.3390/cancers16010173
APA StyleFernández, R., Soza-Ried, C., Iagaru, A., Stephens, A., Müller, A., Schieferstein, H., Sandoval, C., Amaral, H., & Kramer, V. (2024). Imaging GRPr Expression in Metastatic Castration-Resistant Prostate Cancer with [68Ga]Ga-RM2—A Head-to-Head Pilot Comparison with [68Ga]Ga-PSMA-11. Cancers, 16(1), 173. https://doi.org/10.3390/cancers16010173