Multimodal Treatment of Pleural Mesothelioma with Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy: Impact of Additive Chemotherapy
Abstract
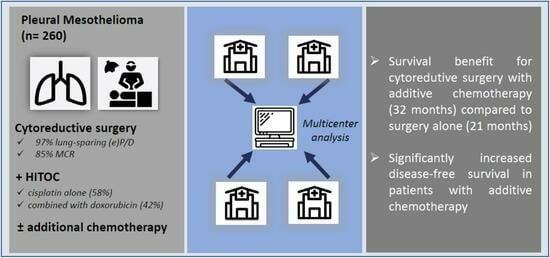
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Definition of Variables
2.3. Statistical Analysis
3. Results
3.1. Surgical Approach
3.2. Postoperative Data
3.3. Multimodal Treatment and Follow-Up
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tedesco, J.; Jaradeh, M.; Vigneswaran, W.T. Malignant Pleural Mesothelioma: Current Understanding of the Immune Microenvironment and Treatments of a Rare Disease. Cancers 2022, 14, 4415. [Google Scholar] [CrossRef] [PubMed]
- Roe, O.D.; Stella, G.M. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Taioli, E.; Wolf, A.S.; Flores, R.M. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann. Thorac. Surg. 2015, 99, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.H.; Recht, A.; Strauss, G.M.; DeCamp, M.M., Jr.; Swanson, S.J.; Liptay, M.J.; Mentzer, S.J.; Sugarbaker, D.J. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann. Thorac. Surg. 1997, 63, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J. Macroscopic complete resection: The goal of primary surgery in multimodality therapy for pleural mesothelioma. J. Thorac. Oncol. 2006, 1, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.V.; Hoffmann, H.; Shah, R.; Eichhorn, F.; Gruenewald, C.; Bulut, E.L.; Griffo, R.; Muley, T.; Christopoulos, P.; Baum, P.; et al. Multimodal therapy of epithelioid pleural mesothelioma: Improved survival by changing the surgical treatment approach. Transl. Lung Cancer Res. 2022, 11, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Gill, R.R.; Yeap, B.Y.; Wolf, A.S.; DaSilva, M.C.; Baldini, E.H.; Bueno, R.; Richards, W.G. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J. Thorac. Cardiovasc. Surg. 2013, 145, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bolukbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., 3rd; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef]
- Cao, C.; Tian, D.; Park, J.; Allan, J.; Pataky, K.A.; Yan, T.D. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014, 83, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ried, M.; Kovacs, J.; Markowiak, T.; Muller, K.; Huppertz, G.; Koller, M.; Winter, H.; Klotz, L.V.; Hatz, R.; Zimmermann, J.; et al. Hyperthermic Intrathoracic Chemotherapy (HITOC) after Cytoreductive Surgery for Pleural Malignancies—A Retrospective, Multicentre Study. Cancers 2021, 13, 4580. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ahern, C.A.; Berlind, C.G.; Lindsay, W.D.; Grover, S.; Friedberg, J.S.; Simone, C.B., 2nd. Treatment of malignant pleural mesothelioma with chemotherapy preceding versus after surgical resection. J. Thorac. Cardiovasc. Surg. 2019, 157, 758–766.e1. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, T.; Koller, M.; Zeman, F.; Huppertz, G.; Hofmann, H.S.; Ried, M.; Group, H.S. Protocol of a retrospective, multicentre observational study on hyperthermic intrathoracic chemotherapy in Germany. BMJ Open 2020, 10, e041511. [Google Scholar] [CrossRef]
- Klotz, L.V.; Gruenewald, C.; Bulut, E.L.; Eichhorn, F.; Thomas, M.; Shah, R.; Kriegsmann, M.; Schmidt, W.; Kofler, O.; Winter, H.; et al. Cytoreductive Thoracic Surgery Combined with Hyperthermic Chemoperfusion for Pleural Malignancies: A Single-Center Experience. Respiration 2021, 100, 1165–1173. [Google Scholar] [CrossRef]
- Bueno, R.; Opitz, I.; Taskforce, I.M. Surgery in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1638–1654. [Google Scholar] [CrossRef]
- Schwartz, R.M.; Watson, A.; Wolf, A.; Flores, R.; Taioli, E. The impact of surgical approach on quality of life for pleural malignant mesothelioma. Ann. Transl. Med. 2017, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.J.; Donahoe, L.; Bradbury, P.A.; Leighl, N.; Keshavjee, S.; Hope, A.; Pal, P.; Cabanero, M.; Czarnecka, K.; McRae, K.; et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): Final results from a single-centre, phase 2 trial. Lancet Oncol. 2021, 22, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Gill, R.R.; Mazzola, E.; Freyaldenhoven, S.; Swanson, S.J.; Jaklitsch, M.T.; Sugarbaker, D.J.; Bueno, R. Pleurectomy Decortication in the Treatment of Malignant Pleural Mesothelioma: Encouraging Results and Novel Prognostic Implications Based on Experience in 355 Consecutive Patients. Ann. Surg. 2022, 275, 1212–1220. [Google Scholar] [CrossRef]
- Ambrogi, M.C.; Bertoglio, P.; Aprile, V.; Chella, A.; Korasidis, S.; Fontanini, G.; Fanucchi, O.; Lucchi, M.; Mussi, A. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: A 10-year experience. J. Thorac. Cardiovasc. Surg. 2017, 155, 1857–1866.e2. [Google Scholar] [CrossRef]
- Sharkey, A.J.; Bilancia, R.; Tenconi, S.; Nakas, A.; Waller, D.A. Extended pleurectomy decortication for malignant pleural mesothelioma in the elderly: The need for an inclusive yet selective approach. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ripley, R.T. Extended Pleurectomy and Decortication for Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020, 30, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.J.; O’Byrne, K.J.; Nakas, A.; Tenconi, S.; Fennell, D.A.; Waller, D.A. How does the timing of chemotherapy affect outcome following radical surgery for malignant pleural mesothelioma? Lung Cancer 2016, 100, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Raskin, J.; Surmont, V.; Cornelissen, R.; Baas, P.; van Schil, P.E.Y.; van Meerbeeck, J.P. A randomized phase II study of pleurectomy/decortication preceded or followed by (neo-)adjuvant chemotherapy in patients with early stage malignant pleural mesothelioma (EORTC 1205). Transl. Lung Cancer Res. 2018, 7, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Stahel, R.A.; Riesterer, O.; Xyrafas, A.; Opitz, I.; Beyeler, M.; Ochsenbein, A.; Fruh, M.; Cathomas, R.; Nackaerts, K.; Peters, S.; et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): A randomised, international, multicentre phase 2 trial. Lancet Oncol. 2015, 16, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Waller, D.A.; Dawson, A.G. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann. Transl. Med. 2017, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhao, S.S.; Ren, M.; Liu, Z.L.; Li, Z.; Yang, L. Effect of hyperthermic intrathoracic chemotherapy on the malignant pleural mesothelioma: A systematic review and meta-analysis. Oncotarget 2017, 8, 100640–100647. [Google Scholar] [CrossRef]
- Sauter, J.L.; Dacic, S.; Galateau-Salle, F.; Attanoos, R.L.; Butnor, K.J.; Churg, A.; Husain, A.N.; Kadota, K.; Khoor, A.; Nicholson, A.G.; et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J. Thorac. Oncol. 2022, 17, 608–622. [Google Scholar] [CrossRef]
- Shah, R.; Klotz, L.V.; Chung, I.; Feißt, M.; Schneider, M.A.; Riedel, J.; Bischoff, H.; Eichhorn, M.E.; Thomas, M. A Phase II Trial of Nivolumab With Chemotherapy Followed by Maintenance Nivolumab in Patients with Pleural Mesothelioma After Surgery: The NICITA Study Protocol. Clin. Lung Cancer 2020, 22, 142–146. [Google Scholar] [CrossRef]



| Clinical Parameters | n = 260 | |
|---|---|---|
| Sex (n, %) | ||
| female | 49 (18.8) | |
| male | 211 (81.2) | |
| Age (years) (mean ± SD) | 65.5 ± 9.0 | |
| BMI (m2/kg) (mean ± SD) | 26.3 ± 3.9 | |
| ECOG (n, %) | ||
| 0 | 159 (61.2) | |
| 1 | 89 (34.2) | |
| 2 | 1 (0.4) | |
| missing | 11 (4.2) | |
| Asbestos exposure (n, %) | ||
| no | 56 (21.5) | |
| yes | 171 (65.8) | |
| missing/unknown | 33 (12.7) | |
| Side (n, %) | ||
| right | 156 (60.0) | |
| left | 104 (40.0) | |
| MPM histology (n, %) | ||
| epithelioid | 220 (84.6) | |
| biphasic | 35 (13.5) | |
| sarcomatoid | 5 (1.9) | |
| MPM tumor stage (UICC) (n, %) | ||
| IA | 15 (5.8) | |
| IB | 110 (42.3) | |
| II | 39 (15.0) | |
| III | 90 (34.6) | |
| IV | 6 (2.3) | |
| Intraoperative Parameters | n = 260 | |
|---|---|---|
| Resection method (n, %) | ||
| P/D | 60 (23.1) | |
| eP/D | 193 (74.2) | |
| EPP | 7 (2.7) | |
| Resection of diaphragm (n, %) | ||
| no | 87 (54.6) | |
| yes | 173 (66.5) | |
| Replacement of diaphragm (n, %) | ||
| no | 193 (74.2) | |
| yes | 67 (25.8) | |
| Resection of pericardium (n, %) | ||
| no | 142 (54.6) | |
| yes | 118 (45.4) | |
| Replacement of pericardium (n, %) | ||
| no | 199 (76.5) | |
| yes | 61 (23.5) | |
| Resection of chest wall (n, %) | ||
| no | 235 (90.4) | |
| yes | 25 (9.6) | |
| Resection status | ||
| MCR | 222 (85.4) | |
| R2 | 38 (14.6) | |
| Blood loss (median, IQR) * | 800 (500, 1200) | |
| Transfusion (n, %) | ||
| no | 112 (43.1) | |
| yes | 145 (55.8) | |
| missing | 3 (1.2) | |
| Chemotherapy substances (n, %) | ||
| cisplatin alone | 152 (58.5) | |
| cisplatin plus doxorubicin | 108 (41.5) | |
| Cisplatin dosage (n, %) | ||
| low dose (≤125 mg/m2 BSA) | 191 (73.2) | |
| high dose (>125 mg/m2 BSA) | 70 (26.8) | |
| Intraoperative complication | ||
| no | 252 (96.9) | |
| yes | 8 (3.1) | |
| Postoperative Parameters | n = 260 | |
|---|---|---|
| Postoperative complications (n, %) | ||
| no | 118 (45.4) | |
| yes/Clavien–Dindo classification | 142 (54.6) | |
| I | 12 (4.6) | |
| II | 52 (20.0) | |
| IIIa | 28 (10.8) | |
| IIIb | 37 (14.2) | |
| IVa | 1 (0.4) | |
| IVb | 3 (1.2) | |
| V | 9 (3.5) | |
| Revision (n, %) | ||
| no | 224 (86.2) | |
| yes/revision due to | 36 (13.8) | |
| coagulothorax | 9 (3.5) | |
| pleural empyema | 3 (1.2) | |
| chylothorax | 5 (1.9) | |
| parenchymal fistula/bronchial fistula | 8 (3.1) | |
| other | 11 (4.2) | |
| Prolonged ventilation > 24 h (n, %) | ||
| no | 251 (96.5) | |
| yes | 9 (3.5) | |
| Extubation in the OR (n, %) | ||
| no | 21 (8.1) | |
| yes | 239 (91.9) | |
| ICU stay [days] (median, IQR) | 2 (1, 4) | |
| Hospital stay [days] (median, IQR) | 20 (15, 29) | |
| In-hospital mortality (n, %) | 9 (3.5) | |
| Postoperative Course | n = 260 | |
|---|---|---|
| Neoadjuvant chemotherapy (n, %) | ||
| no | 197 (75.8) | |
| yes | 63 (24.2) | |
| Adjuvant chemotherapy (n, %) | ||
| no | 171 (65.8) | |
| yes | 86 (33.1) | |
| missing | 3 (1.2) | |
| Additive chemotherapy (n, %) | ||
| no | 121 (46.5) | |
| yes | 136 (52.3) | |
| missing | 3 (1.2) | |
| Adjuvant radiotherapy (n, %) | ||
| no | 199 (76.5) | |
| yes | 58 (22.3) | |
| missing | 3 (1.2) | |
| Recurrence (MCR, n = 222) (n, %) | ||
| no | 52 (23.4) | |
| yes | 170 (76.6) | |
| Progression (R2, n = 38) (n, %) | ||
| no | 13 (34.2) | |
| yes | 38 (65.8) | |
| Location of recurrence/progression (n = 195) (n, %) | ||
| locoregional | 157 (80.5) | |
| distant metastasis | 15 (5.8) | |
| locoregional and distant metastasis | 17 (8.7) | |
| missing | 6 (3.1) | |
| Site of recurrence/progression (n = 195) (n, %) | ||
| ipsilateral | 174 (89.2) | |
| contralateral | 6 (3.1) | |
| missing | 15 (5.8) | |
| Therapy for recurrence/progression (n = 195) (n, %) | ||
| no | 11 (5.6) | |
| unknown | 16 (8.2) | |
| yes/type | 168 (86.2) | |
| surgery | 19 (9.7) | |
| chemotherapy | 117 (60.0) | |
| radiotherapy | 30 (15.4) | |
| supportive care | 14 (7.2) | |
| other | 3 (1.5) | |
| Time between the start of primary therapy until the end of follow-up in months * (reverse Kaplan–Meier OS) (median, 95% CI) | 48 (38, 58) | |
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Sig. | HR | 95% CI | Sig. | HR | 95% CI | |||
| cisplatin dosage | 0.350 | 0.81 | 0.53 | 1.25 | 0.831 | 1.04 | 0.74 | 1.45 |
| chemotherapeutic agent | 0.114 | 1.35 | 0.93 | 1.95 | 0.556 | 1.10 | 0.80 | 1.53 |
| resection status | <0.001 | 2.25 | 1.45 | 3.49 | 0.010 | 1.72 | 1.14 | 2.60 |
| histological subtype | <0.001 | 2.27 | 1.50 | 3.41 | <0.001 | 2.43 | 1.63 | 3.61 |
| UICC stage | 0.544 | 1.11 | 0.79 | 1.57 | 0.986 | 1.00 | 0.74 | 1.34 |
| postoperative AKI | 0.897 | 1.03 | 0.64 | 1.65 | 0.515 | 1.14 | 0.77 | 1.70 |
| additive chemotherapy | 0.184 | 0.79 | 0.56 | 1.12 | 0.003 | 0.64 | 0.47 | 0.85 |
| time between the initial diagnosis and start of primary therapy | 0.712 | 1.01 | 0.97 | 1.05 | 0.772 | 1.00 | 0.98 | 1.04 |
| N = 255 *; n = 162 dead, n = 93 censored | N = 256 **; n = 218 recurrence/progression or dead, n = 38 censored | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotz, L.V.; Zimmermann, J.; Müller, K.; Kovács, J.; Hassan, M.; Koller, M.; Schmid, S.; Huppertz, G.; Markowiak, T.; Passlick, B.; et al. Multimodal Treatment of Pleural Mesothelioma with Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy: Impact of Additive Chemotherapy. Cancers 2024, 16, 1587. https://doi.org/10.3390/cancers16081587
Klotz LV, Zimmermann J, Müller K, Kovács J, Hassan M, Koller M, Schmid S, Huppertz G, Markowiak T, Passlick B, et al. Multimodal Treatment of Pleural Mesothelioma with Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy: Impact of Additive Chemotherapy. Cancers. 2024; 16(8):1587. https://doi.org/10.3390/cancers16081587
Chicago/Turabian StyleKlotz, Laura V., Julia Zimmermann, Karolina Müller, Julia Kovács, Mohamed Hassan, Michael Koller, Severin Schmid, Gunnar Huppertz, Till Markowiak, Bernward Passlick, and et al. 2024. "Multimodal Treatment of Pleural Mesothelioma with Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy: Impact of Additive Chemotherapy" Cancers 16, no. 8: 1587. https://doi.org/10.3390/cancers16081587
APA StyleKlotz, L. V., Zimmermann, J., Müller, K., Kovács, J., Hassan, M., Koller, M., Schmid, S., Huppertz, G., Markowiak, T., Passlick, B., Hofmann, H. -S., Winter, H., Hatz, R. A., Eichhorn, M. E., & Ried, M. (2024). Multimodal Treatment of Pleural Mesothelioma with Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy: Impact of Additive Chemotherapy. Cancers, 16(8), 1587. https://doi.org/10.3390/cancers16081587