Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light
Abstract
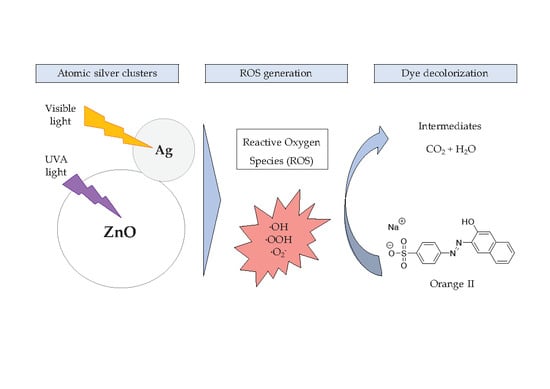
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Influence of Ag Nanoclusters Loading onto Photocatalytic Activity of ZnO
2.3. Influence of Photocatalyst Concentration on OII Removal
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Nanostructured Photocatalysts
3.2.1. ZnO Nanoparticles
3.2.2. ZnO–Ag Nanocomposites
3.3. Characterization of the ZnO–Ag Nanocomposites
3.4. Photocatalytic Degradation of Orange II under UVA and White Light
3.5. Determination of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Sustainability: Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Ahuja, S. Water Reclamation and Sustainability, 1st ed.; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Martín-Pozo, L.; de Alarcón-Gómez, B.; Rodríguez-Gómez, R.; García-Córcoles, M.T.; Çipa, M.; Zafra-Gómez, A. Analytical methods for the determination of emerging contaminants in sewage sludge samples. A review. Talanta 2019, 192, 508–533. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Bakker, J.; Bijlsma, L.; de Boer, J.; Botero-Coy, A.M.; Bruinen de Bruin, Y.; Fischer, S.; Hollender, J.; Kasprzyk-Hordern, B.; Lamoree, M.; et al. The role of analytical chemistry in exposure science: Focus on the aquatic environment. Chemosphere 2019, 222, 564–583. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the catalyst type (TiO2 and ZnO) on the photocatalytic oxidation of pharmaceuticals in the aquatic environment. Desalin. Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Kosera, V.S.; Cruz, T.M.; Chaves, E.S.; Tiburtius, E.R.L. Triclosan degradation by heterogeneous photocatalysis using ZnO immobilized in biopolymer as catalyst. J. Photochem. Photobiol. A Chem. 2017, 344, 184–191. [Google Scholar] [CrossRef]
- Fernández, L.; Borzecka, W.; Lin, Z.; Schneider, R.J.; Huvaere, K.; Esteves, V.I.; Cunha, Â.; Tomé, J.P.C. Nanomagnet-photosensitizer hybrid materials for the degradation of 17β-estradiol in batch and flow modes. Dye Pigment 2017, 142, 535–543. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
- Ameen, S.; Shaheer Akhtar, M.; Shik Shin, H. Speedy photocatalytic degradation of bromophenol dye over ZnO nanoflowers. Mater. Lett. 2017, 209, 150–154. [Google Scholar] [CrossRef]
- Papari, G.P.; Silvestri, B.; Vitiello, G.; De Stefano, L.; Rea, I.; Luciani, G.; Aronne, A.; Andreone, A. Morphological, Structural, and Charge Transfer Properties of F-Doped ZnO: A Spectroscopic Investigation. J. Phys. Chem. C 2017, 121, 16012–16020. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Dharmarajan, R.; Liu, Y.; Naidu, R. Photocatalytic degradation of azo dye acid orange 7 using different light sources over Fe3+-doped TiO2 nanocatalysts. Environ. Technol. Innov. 2018, 12, 27–42. [Google Scholar] [CrossRef]
- Ziashahabi, A.; Prato, M.; Dang, Z.; Poursalehi, R.; Naseri, N. The effect of silver oxidation on the photocatalytic activity of Ag/ZnO hybrid plasmonic/metal-oxide nanostructures under visible light and in the dark. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx-Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Buceta, D.; Piñeiro, Y.; Vázquez-Vázquez, C.; Rivas, J.; López-Quintela, M.A. Metallic clusters: Theoretical background, properties and synthesis in microemulsions. Catalysts 2014, 4, 356–374. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Buceta, D.; Requejo, F.G.; Giovanetti, L.J.; López-Quintela, M.A. Photostability of gold nanoparticles with different shapes: The role of Ag clusters. Nanoscale 2015, 7, 11273–11279. [Google Scholar] [CrossRef]
- Vilar-Vidal, N.; Rey, J.R.; Quintela, M.A.L. Green emitter copper clusters as highly effi cient and reusable visible degradation photocatalysts. Small 2014, 10, 3632–3636. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-Directing and High-Efficiency Photocatalytic Hydrogen Production by Ag Clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Ge, M.; Sahu, B.R.; Tarakeshwar, P.; Kim, K.S. Geometrical and Electronic Structures of Gold, Silver, and Gold−Silver Binary Clusters: Origins of Ductility of Gold and Gold−Silver Alloy Formation. J. Phys. Chem. B 2003, 107, 9994–10005. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.M.; Zhou, Z.W.; Tian, K. Preparation of Ag nanoparticles coated tetrapod-like ZnO whisker photocatalysts using photoreduction. Mater. Sci. Eng. B 2011, 176, 978–983. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.J.; Rojas, H.; Navío, J.A.A.; Hidalgo, M.C.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Chen, P.K.; Lee, G.J.; Anandan, S.; Wu, J.J. Synthesis of ZnO and Au tethered ZnO pyramid-like microflower for photocatalytic degradation of orange II. Mater. Sci. Eng. B 2012, 177, 190–196. [Google Scholar] [CrossRef]
- Lin, J.C.T.; Sopajaree, K.; Jitjanesuwan, T.; Lu, M.C. Application of visible light on copper-doped titanium dioxide catalyzing degradation of chlorophenols. Sep. Purif. Technol. 2018, 191, 233–243. [Google Scholar] [CrossRef]
- Butler, E.B.; Chen, C.C.; Hung, Y.T.; Al Ahmad, M.S.; Fu, Y.P. Effect of Fe-doped TiO2 photocatalysts on the degradation of acid orange 7. Integr. Ferroelectr. 2016, 168, 1–9. [Google Scholar] [CrossRef]
- Siuleiman, S.; Kaneva, N.; Bojinova, A.; Papazova, K.; Apostolov, A.; Dimitrov, D. Photodegradation of Orange II by ZnO and TiO2 powders and nanowire ZnO and ZnO/TiO2 thin films. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 408–413. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Zhou, J.L.; Johir, M.A.H.; Ahmed, M.B. Photocatalysis of estrone in water and wastewater: Comparison between Au-TiO2 nanocomposite and TiO2, and degradation by-products. Sci. Total Environ. 2018, 610–611, 521–530. [Google Scholar] [CrossRef]
- Feldmann, C.; Jungk, H.O. Polyol-Mediated Preparation of Nanoscale Oxide Particles. Angew. Chem. Int. Ed. 2001, 40, 359–362. [Google Scholar] [CrossRef]





| Irradiation Source | [Catalyst] (mg L−1) | ZnO–Ag (1.3%) | ZnO | ||||
|---|---|---|---|---|---|---|---|
| R2 | k (h1) | t1/2 (h) | R2 | k (h−1) | t1/2 (h) | ||
| White light | 200 | 0.9861 | 0.235 ± 0.006 | 2.96 | 0.9425 | 0.036 ± 0.002 | 19.25 |
| 350 | 0.9862 | 0.290 ± 0.007 | 2.41 | 0.9728 | 0.083 ± 0.003 | 8.25 | |
| 500 | 0.9930 | 0.390 ± 0.007 | 1.78 | 0.9847 | 0.089 ± 0.002 | 7.70 | |
| 750 | 0.9624 | 0.455 ± 0.017 | 1.52 | 0.9748 | 0.141 ± 0.005 | 4.81 | |
| 1000 | 0.9984 | 0.308 ± 0.003 | 2.27 | 0.9635 | 0.121 ± 0.004 | 5.78 | |
| UVA light | 50 | 0.9859 | 0.541 ± 0.018 | 1.28 | 0.9323 | 0.346 ± 0.023 | 1.99 |
| 100 | 0.9854 | 0.758 ± 0.026 | 0.92 | 0.9923 | 0.311 ± 0.008 | 2.22 | |
| 150 | 0.9823 | 1.006 ± 0.037 | 0.69 | 0.9988 | 0.388 ± 0.004 | 1.78 | |
| 200 | 0.9722 | 3.436 ± 0.176 | 0.20 | 0.9990 | 0.552 ± 0.005 | 1.26 | |
| 350 | 0.9778 | 3.019 ± 0.137 | 0.23 | 0.9951 | 0.633 a ± 0.012 | 1.10 | |
| 500 | 0.9946 | 2.650 ± 0.055 | 0.26 | 0.9925 | 0.642 a ± 0.016 | 1.08 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, J.; Fernández, L.; Bava, Y.B.; Buceta, D.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Feijoo, G.; Moreira, M.T. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts 2020, 10, 31. https://doi.org/10.3390/catal10010031
González-Rodríguez J, Fernández L, Bava YB, Buceta D, Vázquez-Vázquez C, López-Quintela MA, Feijoo G, Moreira MT. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts. 2020; 10(1):31. https://doi.org/10.3390/catal10010031
Chicago/Turabian StyleGonzález-Rodríguez, Jorge, Lucía Fernández, Yanina B. Bava, David Buceta, Carlos Vázquez-Vázquez, Manuel Arturo López-Quintela, Gumersindo Feijoo, and Maria Teresa Moreira. 2020. "Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light" Catalysts 10, no. 1: 31. https://doi.org/10.3390/catal10010031
APA StyleGonzález-Rodríguez, J., Fernández, L., Bava, Y. B., Buceta, D., Vázquez-Vázquez, C., López-Quintela, M. A., Feijoo, G., & Moreira, M. T. (2020). Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts, 10(1), 31. https://doi.org/10.3390/catal10010031