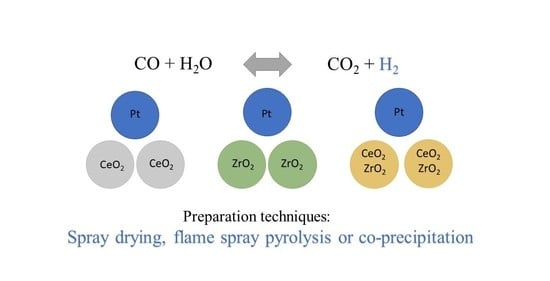
Water–Gas Shift Activity of Pt Catalysts Prepared by Different Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Activity Measurements
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Holladay, J.D.; Wang, Y.; Jones, E. Review of Developments in Portable Hydrogen Production Using Microreactor Technology. Chem. Rev. 2004, 104, 4767–4790. [Google Scholar] [CrossRef]
- Ladebeck, J.R.; Wagner, J.P. Handbook of Fuel Cells—Fundamentals, Technology and Applications, Vol. 3: Fuel Cell Technology and Applications; Vielstich, W., Gasteiger, H.A., Lamm, A., Eds.; Wiley and Sons: New York, NY, USA, 2003; pp. 190–201. [Google Scholar]
- Palma, V.; Ruocco, C.; Cortese, M.; Renda, S.; Meloni, E.; Festa, G.; Martino, M. Platinum Based Catalysts in the Water Gas Shift Reaction: Recent Advances. Metals 2020, 10, 866. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.; Graham, G. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Zalc, J.; Sokolovskii, V.; Löffler, D. Are Noble Metal-Based Water–Gas Shift Catalysts Practical for Automotive Fuel Processing? J. Catal. 2002, 206, 169–171. [Google Scholar] [CrossRef]
- Wang, X.; Gorte, R.J.; Wagner, J. Deactivation Mechanisms for Pd/Ceria during the Water–Gas-Shift Reaction. J. Catal. 2002, 212, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Kašpar, J.; Fornasiero, P. Nanostructured materials for advanced automotive de-pollution catalysts. J. Solid State Chem. 2003, 171, 19–29. [Google Scholar] [CrossRef]
- Ozawa, M. Role of cerium–zirconium mixed oxides as catalysts for car pollution: A short review. J. Alloy. Compd. 1998, 275–277, 886–890. [Google Scholar] [CrossRef]
- Ricote, S.; Jacobs, G.; Milling, M.; Ji, Y.; Patterson, P.M.; Davis, B.H. Characterization and testing of binary mixed oxides of ceria and zirconia promoted with Pt. Appl. Catal. A Gen. 2006, 303, 35–47. [Google Scholar] [CrossRef]
- Letichevsky, S.; Tellez, C.A.; De Avillez, R.R.; Da Silva, M.I.P.; Fraga, M.A.; Appel, L.G. Obtaining CeO2–ZrO2 mixed oxides by coprecipitation: Role of preparation conditions. Appl. Catal. B Environ. 2005, 58, 203–210. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Salama, T.; Othman, A.; El-Shobaky, G. Low temperature water-gas shift reaction on cerium containing mordenites prepared by different methods. Appl. Catal. A Gen. 2005, 279, 23–33. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Sobczak, J.W.; Idakiev, V.; Andreeva, D. Effect of synthesis procedure on the low-temperature WGS activity of Au/ceria catalysts. Appl. Catal. B Environ. 2004, 49, 73–81. [Google Scholar] [CrossRef]
- Zerva, C.; Philippopoulos, C. Ceria catalysts for water gas shift reaction: Influence of preparation method on their activity. Appl. Catal. B Environ. 2006, 67, 105–112. [Google Scholar] [CrossRef]
- Jensen, J.R. Flame Synthesis of Composite Oxides for Catalytic Applications. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2001. [Google Scholar]
- Hansen, J.P.; Jensen, J.R.; Livbjerg, H.; Johannessen, T. Synthesis of ZnO particles in a quench-cooled flame reactor. AIChE J. 2001, 47, 2413–2418. [Google Scholar] [CrossRef]
- Johannessen, T.; Jensen, J.; Mosleh, M.; Johansen, J.; Quaade, U.; Livbjerg, H. Flame Synthesis of Nanoparticles: Applications in Catalysis and Product/Process Engineering. Chem. Eng. Res. Des. 2004, 82, 1444–1452. [Google Scholar] [CrossRef]
- Meland, H.; Johannessen, T.; Arstad, B.; Venvik, H.J.; Rønning, M.; Holmén, A. Preparation of low temperature water-gas shift catalysts by flame spray pyrolysis. Stud. Surf. Sci. Catal. 2006, 162, 985–992. [Google Scholar]
- Lee, D.; Ha, G.; Kim, B.K. Synthesis of Cu-Al2O3 nano composite powder. Scr. Mater. 2001, 44, 2137–2140. [Google Scholar] [CrossRef]
- Robertz, B.; Boschini, F.; Rulmont, A.; Cloots, R.; Van Driessche, I.; Hoste, S.; Lecomte-Beckers, J. Preparation of BaZrO3 powders by a spray-drying process. J. Mater. Res. 2003, 18, 1325–1332. [Google Scholar] [CrossRef]
- Nelson, N.C.; Szanyi, J. Heterolytic Hydrogen Activation: Understanding Support Effects in Water–Gas Shift, Hydrodeoxygenation, and CO Oxidation Catalysis. ACS Catal. 2020, 10, 5663–5671. [Google Scholar] [CrossRef]
- Ginés, M.; Amadeo, N.; Laborde, M.; Apesteguía, C. Activity and structure-sensitivity of the water-gas shift reaction over Cu, Zn, Al mixed oxide catalysts. Appl. Catal. A Gen. 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Saito, M.; Wu, J.; Tomoda, K.; Takahara, I.; Murata, K. Effects of ZnO Contained in Supported Cu-Based Catalysts on Their Activities for Several Reactions. Catal. Lett. 2002, 83, 1–4. [Google Scholar] [CrossRef]
- Saito, M.; Tomoda, K.; Takahara, I.; Murata, K.; Inaba, M. Effects of Pretreatments of Cu/ZnO-Based Catalysts on Their Activities for the Water–Gas Shift Reaction. Catal. Lett. 2003, 89, 11–13. [Google Scholar] [CrossRef]
- Choung, S.Y.; Ferrandon, M.; Krause, T. Pt-Re bimetallic supported on CeO2-ZrO2 mixed oxides as water–gas shift catalysts. Catal. Today 2005, 99, 257–262. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Hou, X.; Wu, N.; Sun, Y.; Li, Z.; Wu, Z. Small-angle X-ray scattering study on the microstructure evolution of zirconia nanoparticles during calcination. J. Solid State Chem. 2006, 179, 959–967. [Google Scholar] [CrossRef]
- Ksapabutr, B.; Gulari, E.; Wongkasemjit, S. Preparation of zirconia powders by sol–gel route of sodium glycozirconate complex. Powder Technol. 2004, 148, 11–14. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. Tetragonal nanocrystalline zirconia powder with high surface area and mesoporous structure. Powder Technol. 2006, 168, 59–63. [Google Scholar] [CrossRef]
- Devassy, B.M.; Halligudi, S. Effect of calcination temperature on the catalytic activity of zirconia-supported heteropoly acids. J. Mol. Catal. A Chem. 2006, 253, 8–15. [Google Scholar] [CrossRef]
- Fan, J.; Wu, X.; Ran, R.; Weng, D. Influence of the oxidative/reductive treatments on the activity of Pt/Ce0.67Zr0.33O2 catalyst. Appl. Surf. Sci. 2005, 245, 162–171. [Google Scholar] [CrossRef]
- Seo, D.J.; Park, S.B. Preparation of ferrite nanoparticles by flame-assisted spray pyrolysis. In Proceedings of the European Aerosol Conference 2003, Madrid, Spain, 31 August–5 September 2003. [Google Scholar]
- Chenu, E.; Jacobs, G.; Crawford, A.C.; Keogh, R.A.; Patterson, P.M.; Sparks, D.E.; Davis, B.H. Water-gas shift: An examination of Pt promoted MgO and tetragonal and monoclinic ZrO2 by in situ drifts. Appl. Catal. B Environ. 2005, 59, 45–56. [Google Scholar] [CrossRef]
- Perez-Hernandez, R.; Aguilar, F.; Gómez-Cortés, A.; Diaz, G. NO reduction with CH4 or CO on Pt/ZrO2–CeO2 catalysts. Catal. Today 2005, 107–108, 175–180. [Google Scholar] [CrossRef]
- Azzam, K.; Babich, I.; Seshan, K.; Lefferts, L. Bifunctional catalysts for single-stage water–gas shift reaction in fuel cell applications. Part 1. Effect of the support on the reaction sequence. J. Catal. 2007, 251, 153–162. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T.; Konarides, D.I. Water–gas shift activity of doped Pt/CeO2 catalysts. Chem. Eng. J. 2007, 134, 16–22. [Google Scholar] [CrossRef]
- Querino, P.S.; Bispo, J.R.C.; Rangel, M. The effect of cerium on the properties of Pt/ZrO2 catalysts in the WGSR. Catal. Today 2005, 107–108, 920–925. [Google Scholar] [CrossRef]
- Passos, F.B.; De Oliveira, E.R.; Mattos, L.V.; Noronha, F.B. Partial oxidation of methane to synthesis gas on Pt/CexZr1−xO2 catalysts: The effect of the support reducibility and of the metal dispersion on the stability of the catalysts. Catal. Today 2005, 101, 23–30. [Google Scholar] [CrossRef]
- Mikulová, J.; Rossignol, S.; Barbier, J.; Duprez, D., Jr.; Kappenstein, C. Characterizations of platinum catalysts supported on Ce, Zr, Pr-oxides and formation of carbonate species in catalytic wet air oxidation of acetic acid. Catal. Today 2007, 124, 185–190. [Google Scholar] [CrossRef]
- Ayastuy, J.; González-Marcos, M.; Gil-Rodríguez, A.; González-Velasco, J.; Gutiérrez-Ortiz, M.A. Selective CO oxidation over CeXZr1−XO2-supported Pt catalysts. Catal. Today 2006, 116, 391–399. [Google Scholar] [CrossRef]
- Mattos, L.V.; Noronha, F.B. Partial oxidation of ethanol on supported Pt catalysts. J. Power Sources 2005, 145, 10–15. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.; Andreeva, D.; Mitov, I. Influence of the microscopic properties of the support on the catalytic activity of Au/ZnO, Au/ZrO2, Au/Fe2O3, Au/Fe2O3–ZnO, Au/Fe2O3–ZrO2 catalysts for the WGS reaction. Appl. Catal. A Gen. 2000, 202, 91–97. [Google Scholar] [CrossRef]
- Huber, F.; Venvik, H.J.; Ronning, M.; Walmsley, J.C.; Holmen, A. Preparation and characterization of nanocrystalline, high-surface area Cu, Ce, Zr mixed oxide catalysts from homogeneous co-precipitation. Chem. Eng. J. 2008, 137, 686–702. [Google Scholar] [CrossRef]
- Huber, F.; Yu, Z.; Walmsley, J.C.; Chen, D.; Venvik, H.J.; Holmen, A. Nanocrystalline Cu-Ce-Zr mixed oxide catalysts for water-gas shift: Carbon nanofibers as dispersing agent for the mixed oxide particles. Appl. Catal. B Environ. 2007, 71, 7–15. [Google Scholar] [CrossRef]
- Johannessen, T.; Johansen, J.; Mosleh, M.; Thybo, S.; Quaade, U.; Jensen, J.R.; Livbjerg, H. Application of nano-particles produced by flame aerosol methods. In Proceedings of the International Congress for Particle Technology (PARTEC 2004), Nuremberg, Germany, 16–18 March 2004. [Google Scholar]
- Thybo, S.; Jensen, S.; Johansen, J.; Johannessen, T.; Hansen, O.; Quaade, U.J. Flame spray deposition of porous catalysts on surfaces and in microsystems. J. Catal. 2004, 223, 271–277. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W. X-ray Diffraction. In Spectroscopy in Catalysis, An Introduction; Wiley-VCH: Weinheim, Germany, 2000; pp. 138–145. [Google Scholar]



| Catalyst | SBET (m2/g) | DPt (-) | Dsupport a (nm) | DPt b (nm) |
|---|---|---|---|---|
| Pt/CeO2_CP | 127 | 0.49 | 8 | 2.2 |
| Pt/CeO2_SD | 106 | 0.24 | 8 | 4.6 |
| Pt/CeO2_FSP | 201 | 0.64 | 10 | 1.7 |
| Pt/CeO2/ZrO2_CP | 104 | 0.15 | 7 c | 7.3 |
| Pt/CeO2/ZrO2_SD | 142 | 0.43 | 6 c | 2.6 |
| Pt/CeO2/ZrO2_FSP | 150 | 0.3 | 8 | 3.7 |
| Pt/ZrO2_CP | 70 | 0.03 | 10 | 36.7 |
| Pt/ZrO2_SD | 2 | 0 | - | - |
| Pt/ZrO2_FSP | 118 | 0.14 | 13 | 7.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørkan, H.; Rønning, M.; Venvik, H.J.; Johannessen, T.; Holmen, A. Water–Gas Shift Activity of Pt Catalysts Prepared by Different Methods. Catalysts 2020, 10, 1132. https://doi.org/10.3390/catal10101132
Bjørkan H, Rønning M, Venvik HJ, Johannessen T, Holmen A. Water–Gas Shift Activity of Pt Catalysts Prepared by Different Methods. Catalysts. 2020; 10(10):1132. https://doi.org/10.3390/catal10101132
Chicago/Turabian StyleBjørkan, Hilde, Magnus Rønning, Hilde J. Venvik, Tue Johannessen, and Anders Holmen. 2020. "Water–Gas Shift Activity of Pt Catalysts Prepared by Different Methods" Catalysts 10, no. 10: 1132. https://doi.org/10.3390/catal10101132
APA StyleBjørkan, H., Rønning, M., Venvik, H. J., Johannessen, T., & Holmen, A. (2020). Water–Gas Shift Activity of Pt Catalysts Prepared by Different Methods. Catalysts, 10(10), 1132. https://doi.org/10.3390/catal10101132