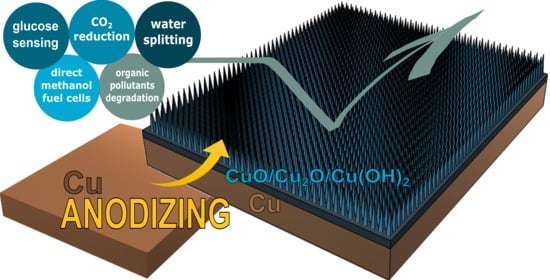
Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions
Abstract
:1. Introduction
- Fixed stoichiometry (despite some minor fluctuations of the composition associated with the anions incorporation);
- Consist of nanopores or nanotubes (except ZnO which is made of nanorods [45]).
2. Anodic Copper Oxides—Unique Features and Applications
2.1. Morphology and Composition of Nanostructures Grown by Copper Anodizing
- Passivation of copper using potentiostat/galvanostat;
- Anodization of copper employing standard 2-electrode system.
2.2. Applications of Electrochemically Grown Copper Oxides
3. Electrochemical CO2 Reduction Reaction

4. Direct Methanol Fuel Cell
5. Glucose Sensing
6. Organic Pollutant Photodegradation
7. Copper Oxides Photocathodes for Photo-Electrochemical (PEC) Water-Splitting Applications
8. Summary
- Copper and copper-derived nanostructures display unique catalytic properties in electrochemical CO2 reduction reaction: copper is the only pure metal that allows C2+ hydrocarbons and alcohols production; such catalysts possess moderately negative adsorption energy for *CO and slightly positive adsorption energy for *H—adsorption of *CO and consequently CO2RR is preferred over HER.
- Oxide-derived copper catalysts possess superb affinity towards C2+ formation during CO2RR, even when compared to bare copper; it prerequisites anodic copper oxides for applications in CO2RR;
- Anodic copper oxides successfully contribute as catalysts in direct methanol fuel cells (DMFCs) —for these materials the highest value of turnover frequency (TOF) in methanol oxidation for non-precious metal was reported, reaching 3.5k s−1 at the vortex potential of 0.65 V vs. Hg|HgO, OH−;
- Anodic copper oxides based glucose sensor achieve very high sensitivity due to their supreme electrocatalytic activity attributed to Cu(II)/Cu(III) redox couple, making the electrode highly sensitive and highly developed surface nanostructured materials;
- High surface area and band structure of the nanostructured anodic copper oxides contribute in photocatalytic degradation of water pollutants;
- Photoelectrochemical water splitting on the nanostructured anodic copper oxides has satisfactory performance due to the formed direct pathways for the photogenerated charge and highly-developed surface area; Cu2O-CuO heterostructures provide rapid charge carrier separation, reducing the recombination of the photogenerated electrons.
Author Contributions
Funding
Conflicts of Interest
References
- Abrahami, S.T.; Hauffman, T.; de Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Adhesive bonding and corrosion performance investigated as a function of aluminum oxide chemistry and adhesives. Corrosion 2017, 73, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kawahara, K.; Kikuchi, T.; Suzuki, R.O.; Natsui, S. Corrosion-resistant porous alumina formed via anodizing aluminum in etidronic acid and its pore-sealing behavior in boiling water. J. Electrochem. Soc. 2019, 166, C261–C269. [Google Scholar] [CrossRef]
- González-Rovira, L.; González-Souto, L.; Astola, P.J.; Bravo-Benítez, C.; Botana, F.J. Assessment of the corrosion resistance of self-ordered anodic aluminum oxide (AAO) obtained in tartaric-sulfuric acid (TSA). Surf. Coat. Technol. 2020, 399, 126131. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Furneaux, R.C.; Rigby, W.R.; Davidson, A.P. The formation of controlled-porosity membranes from anodically oxidized aluminium. Nature 1989, 337, 147–149. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Yoshioka, T.; Matsushima, H.; Ueda, M.; Suzuki, R.O.; Natsui, S.A. Superhydrophilic aluminum surface with fast water evaporation based on anodic alumina bundle structures via anodizing in pyrophosphoric acid. Materials 2019, 12, 3497. [Google Scholar] [CrossRef] [Green Version]
- Norek, M.; Krasiński, A. Controlling of water wettability by structural and chemical modification of porous anodic alumina (PAA): Towards super-hydrophobic surfaces. Surf. Coat. Technol. 2015, 276, 464–470. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.-P. Surface wettability of macroporous anodized aluminum oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef]
- Brudzisz, A.; Rajska, D.; Gajewska, M.; Sulka, G.D.; Brzózka, A. Controlled synthesis and characterization of AgPd nanowire arrays for electrocatalytic applications. J. Electroanal. Chem. 2020, 873, 114373. [Google Scholar] [CrossRef]
- Date, M.K.; Yang, L.-H.; Yang, T.-Y.; Wang, K.-Y.; Su, T.-Y.; Wu, D.-C.; Cheuh, Y.-L. Three-dimensional CuO/TiO2 hybrid nanorod arrays prepared by electrodeposition in AAO membranes as an excellent Fenton-like photocatalyst for dye degradation. Nanoscale Res. Lett. 2020, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Tian, Y.; Wang, C.; Hang, C.; Zhang, H.; Huang, Y.; Zheng, Z. One-step fabrication of copper nanopillar array-filled AAO films by pulse electrodeposition for anisotropic thermal conductive interconnectors. ACS Omega 2019, 4, 6092–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, M.; Reverberi, A.P.; Baino, F. Nanoscale topographical characterization of orbital implant materials. Materials 2018, 11, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical applications of anodic porous alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Maher, S.; Mazinani, A.; Barati, M.R.; Losic, D. Engineered titanium implants for localized drug delivery: Recent advances and perspectives of titania nanotubes arrays. Expert Opin. Drug Deliv. 2018, 15, 1021–1037. [Google Scholar] [CrossRef]
- Bariana, M.; Kaidonis, J.A.; Losic, D.; Ranjitkar, S.; Anderson, P.J. Titania nanotube-based protein delivery system to inhibit cranial bone regeneration in Crouzon model of craniosynostosis. Int. J. Nanomed. 2019, 14, 6313–6324. [Google Scholar] [CrossRef] [Green Version]
- Scisco, G.P.; Haynes, K.; Jones, K.S.; Ziegler, K.J. Single step bonding of thick anodized aluminum oxide templates to silicon wafers for enhanced system-on-a-chip performance. J. Power Sources 2020, 474, 228643. [Google Scholar] [CrossRef]
- Acosta, L.K.; Bertó-Roselló, F.; Xifre-Perez, E.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Stacked nanoporous anodic alumina gradient-index filters with tunable multispectral photonic stopbands as sensing platforms. ACS Appl. Mater. Interfaces 2019, 11, 3360–3371. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Voelcker, N.H.; Santos, A. Nanoporous anodic alumina photonic crystals for optical chemo-and biosensing: Fundamentals, advances, and perspectives. Nanomaterials 2019, 8, 788. [Google Scholar] [CrossRef] [Green Version]
- Ashurov, M.; Gorelik, V.; Napolskii, K.; Klimonsky, S. Anodic alumina photonic crystals as refractive index sensors for controlling the composition of liquid mixtures. Phot. Sens. 2020, 10, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicka, E.; Szultka-Młyńska, M.; Buszewski, B.; Sulka, G.D. Epinephrine sensing at nanostructured Au electrode and determination its oxidative metabolism. Sens. Actuators B Chem. 2016, 237, 206–215. [Google Scholar] [CrossRef]
- Sadykov, A.I.; Kushnir, S.E.; Sapoletova, N.A.; Ivanov, V.K.; Napolskii, K.S. Anodic titania photonic crystals with high reflectance within photonic band gap via pore shape engineering. Script. Mater. 2020, 178, 13–17. [Google Scholar] [CrossRef]
- Hu, H.; Xu, C.; Zhao, Y.; Ziegler, K.J.; Chung, J.N. Boiling and quenching heat transfer advancement by nanoscale surface modification. Sci. Rep. 2017, 7, 6117. [Google Scholar] [CrossRef]
- Hu, H.; Xu, C.; Zhao, Y.; Shaeffer, R.; Ziegler, K.J.; Chung, J.N. Modification and enhancement of cryogenic quenching heat transfer by a nanoporous surface. Int. J. Heat Mass Transf. 2015, 80, 636–643. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Domaschke, M.; Denisov, N.; Fehn, D.; Hwang, I.; Kaufmann, M.; Kunstmann, B.; Schmidt, J.; Meyer, K.; Peukert, W.; et al. Magnéli phases doped with Pt for photocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2019, 2, 8399–8404. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Zhou, X.; Denisov, N.; Yoo, J.; Fehn, D.; Liu, N.; Meyer, K.; Schmuki, P. Self-enhancing H2 evolution from TiO2 nanostructures under illumination. ChemSusChem 2019, 12, 1900–1905. [Google Scholar] [CrossRef] [Green Version]
- Bashirom, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Comparison of ZrO2, TiO2, and α-Fe2O3 nanotube arrays on Cr(VI) photoreduction fabricated by anodization of Zr, Ti, and Fe foils. Mater. Res. Express 2020, 7, 055013. [Google Scholar] [CrossRef]
- Bashirom, N.; Razak, K.A.; Lockman, Z. Synthesis of freestanding amorphous ZrO2 nanotubes by anodization and their application in photoreduction of Cr(VI) under visible light. Surf. Coat. Technol. 2017, 320, 371–376. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, C.; Liu, L.; Teng, X.; Wu, Y.; Hu, W. Electrochemically prepared cuprous oxide film for photo-catalytic oxygen evolution from water oxidation under visible light. Sol. Energy Mater. Sol. Cells 2015, 132, 275–281. [Google Scholar] [CrossRef]
- Shu, X.; Zheng, H.; Xu, G.; Zhao, J.; Cui, L.; Qin, Y.; Wang, Y.; Zhang, Y.; Wu, Y. The anodization synthesis of copper oxide nanosheet arrays and their photoelectrochemical properties. Appl. Surf. Sci. 2017, 412, 505–516. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, S.; Seong, M.; Yoo, H.; Choi, J. NH4-doped anodic WO3 prepared through anodization and subsequent NH4OH treatment for water splitting. Appl. Surf. Sci. 2015, 324, 414–418. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of porous tungsten oxide via anodizing in an ammonium nitrate/ethylene glycol/water mixture for visible light-driven photocatalyst. Appl. Surf. Sci. 2017, 422, 130–137. [Google Scholar] [CrossRef]
- Pisarek, M.; Krajczewski, J.; Wierzbicka, E.; Holdynski, M.; Sulka, G.D.; Nowakowski, R.; Kudelski, A.; Janik-Czachor, M. Influence of the silver deposition method on the activity of platforms for chemometric surface-enhanced Raman scattering measurements: Silver films on ZrO2 nanopore arrays. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 182, 124–129. [Google Scholar] [CrossRef]
- Ambroziak, R.; Hołdyński, M.; Płociński, T.; Pisarek, M.; Kudelski, A. Cubic silver nanoparticles fixed on TiO2 nanotubes as simple and efficient substrates for surface enhanced Raman scattering. Materials 2019, 12, 3373. [Google Scholar] [CrossRef] [Green Version]
- Celik, M.; Buyukserin, F. The use of anodized alumina molds for the fabrication of polymer nanopillar arrays as SERS substrates with tunable properties. Vibrat. Spectrosc. 2019, 104, 102965. [Google Scholar] [CrossRef]
- Nyein, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. TiO2 nanotube arrays formation in fluoride/ethylene glycol electrolyte containing LiOH or KOH as photoanode for dye-sensitized solar cell. J. Photochem. Photobiol. A 2017, 343, 33–39. [Google Scholar] [CrossRef]
- Nyein, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Anodic Ag/TiO2 nanotube array formation in NaOH/fluoride/ethylene glycol electrolyte as a photoanode for dye-sensitized solar cells. Nanotechnology 2016, 27, 355605. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Polymer nanoimprinting using an anodized aluminum mold for structural coloration. Appl. Surf. Sci. 2015, 341, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Pashchanka, M.; Yadav, S.; Cottre, T.; Schneider, J.J. Porous alumina-metallic Pt/Pd, Cr or Al layered nanocoatings with fully controlled variable interference colors. Nanoscale 2014, 6, 12877–12883. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Sieber, I.; Schmuki, P. Self-organized high-aspect-ratio nanoporous zirconium oxides prepared by electrochemical anodization. Small 2005, 1, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Schmuki, P. Fabrication and characterization of smooth high aspect ratio zirconia nanotubes. Chem. Phys. Lett. 2005, 410, 188–191. [Google Scholar] [CrossRef]
- Chilimoniuk, P.; Socha, R.P.; Czujko, T. Nanoporous anodic aluminum-iron oxide with a tunable band gap formed on the FeAl3 intermetallic phase. Materials 2020, 13, 3471. [Google Scholar] [CrossRef]
- Chilimoniuk, P.; Michalska-Domańska, M.; Czujko, T. Formation of nanoporous mixed aluminum-iron oxides by self-organized anodizing of FeAl3 intermetallic alloy. Materials 2019, 12, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.H.; Berenov, A.; Qi, X.; Kappers, M.J.; Barber, Z.H.; Illy, B.; Lockman, Z.; Ryan, M.P.; MacManus-Driscoll, J.L. Electrochemical growth of ZnO nano-rods on polycrystalline Zn foil. Nanotechnology 2003, 14, 968–973. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Misiolek, W.Z. Review of fabrication methods, physical properties, and applications of nanostructured copper oxides formed via electrochemical oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for copper at 25 to 300 °C. J. Electrochem. Soc. 1997, 144, 3476–3483. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Yoo, H.; Choi, J.; Norek, M.; Jóźwik, P.; Misiolek, W.Z. Fabrication and characterization of oxide nano-needles formed by copper passivation in sodium hydroxide solution. Thin Solid Films 2019, 671, 111–119. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Yoo, H.; Choi, J.; Chilimoniuk, P.; Karczewski, K.; Czujko, T. Investigation of oxide nanowires growth on copper via passivation in NaOH aqueous solution. Surf. Interfaces 2019, 14, 15–18. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Stojadinovic, S.; Vasilic, R.; Tadic, N.; Karczewski, K.; Abrahami, S.T.; Buijnsters, J.G.; Mol, J.M.C. Morphology and photoluminescence of nanostructured oxides grown by copper passivation in aqueous potassium hydroxide solution. Mater. Lett. 2017, 198, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Yuan, S.; Liang, B.; Li, G.; Pehkonen, S.O.; Zhang, T.J. Superhydrophobic CuO nanoneedle-covered copper surfaces for anticorrosion. J. Mater. Chem. A 2015, 3, 4374–4388. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Electrochemical study of bulk and monolayer copper in alkaline solution. J. Electrochem. Soc. 2016, 163, H252–H259. [Google Scholar] [CrossRef]
- Cheng, Z.; Ming, D.; Fu, K.; Zhang, N.; Sun, K. pH-controllable water permeation through a nanostructured copper mesh film. ACS Appl. Mater. Interfaces 2012, 4, 5826–5832. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Bai, H.; Zhang, J.; Chen, F.; Shi, G. Copper hydroxide nanoneedle and nanotube arrays fabricated by anodization of copper. J. Phys. Chem. B 2005, 109, 22836–22842. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, Y.; Wang, X.; Wang, Q. Electrochemical formation and reduction of copper oxide nanostructures in alkaline media. Electrochem. Commun. 2013, 36, 99102. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Electrochemical fabrication of complex copper oxide nanoarchitectures via copper anodization in aqueous and non-aqueous electrolytes. Mater. Lett. 2011, 65, 1949–1955. [Google Scholar] [CrossRef]
- Jiang, W.; He, J.; Xiao, F.; Yuan, S.; Lu, H.; Liang, B. Preparation and antiscaling application of superhydrophobic anodized CuO nanowire surfaces. Ind. Eng. Chem. Res. 2015, 54, 6874–6883. [Google Scholar] [CrossRef]
- Xie, J.F.; Huang, Y.X.; Li, W.W.; Song, X.N.; Xiong, L.; Yu, H.Q. Efficient electrochemical CO2 reduction on a unique chrysanthemum-like Cu nanoflower electrode and direct observation of carbon deposite. Electrochim. Acta 2014, 139, 137144. [Google Scholar] [CrossRef]
- Oyarzún Jerez, D.P.; López Teijelo, M.; Ramos Cervantes, W.; Linarez Pérez, O.E.; Sánchez, J.; Pizarro, G.C.; Acosta, G.; Flores, M.; Arratia-Perez, R. Nanostructuring of anodic copper oxides in fluoride-containing ethylene glycol media. J. Electroanal. Chem. 2017, 807, 181186. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Paliwoda, D.; Abrahami, S.T.; Michalska-Domanska, M.; Landskron, K.; Buijnsters, J.G.; Mol, J.M.C.; Terryn, H.; Misiolek, W.Z. Nanorods grown by copper anodizing in sodium carbonate. J. Electroanal. Chem. 2020, 857, 113628. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Paliwoda, D.; Chen, Z.; Landskron, K.; Misiolek, W.Z. Hard anodization of copper in potassium carbonate aqueous solution. Mater. Lett. 2019, 252, 182–185. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Misiołek, W.Z. Nanostructured anodic films grown on copper: A review of fabrication techniques and applications. In Nanostructured Anodic Metals Oxides. Synthesis and Applications, 1st ed.; Sulka, G.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 415–452. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Wang, K.-K.; Chandrasekar, S.; Paliwoda, D.; Nowak-Stępniowska, A.; Misiolek, W.Z. The impact of ethylenediaminetetraacetic acid (EDTA) additive on anodization of copper in KHCO3–hindering Cu2+ re-deposition by EDTA influences morphology and composition of the nanostructures. J. Electroanal. Chem. 2020, 871, 114245. [Google Scholar] [CrossRef]
- Le Coz, F.; Arurault, L.; Datas, L. Chemical analysis of a single basic cell of porous anodic aluminium oxide templates. Mater. Character. 2010, 61, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Exploration for the self-ordering of porous alumina fabricated via anodizing in etidronic acid. Electrochim. Acta 2016, 211, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Ng, Y.H.; Amal, R. Embedment of anodized p-type Cu2O thin films with CuO nanowires for improvement in photoelectrochemical stability. Nanoscale 2013, 5, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P. Highly stable copper oxide composite as an effective photocathode for water splitting via a facile electrochemical synthesis strategy. J. Mater. Chem. 2012, 22, 2456–2464. [Google Scholar] [CrossRef]
- Arurault, L.; Belghith, M.H.; Bes, R.S. Manganese pigmented anodized copper as solar selective absorber. J. Mater. Sci. 2007, 42, 1190–1195. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.H.; Suryanto, S.; Al Hazza, M.H.F.; Haidera, F.I. Developing of corrosion resistance nano copper oxide coating on copper using anodization in oxalate solution. Int. J. Eng. Trans. C 2018, 31, 450–455. [Google Scholar]
- Ratynski, M.; Hamankiewicz, B.; Krajewski, M.; Boczar, M.; Ziolkowska, D.; Czerwinski, A. Single step, electrochemical preparation of copper-based positive electrode for lithium primary cells. Materials 2018, 11, 2126. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, Y.; Xiong, H.; Qin, C.; Zhao, W.; Liu, X. Yucca fern shaped CuO nanowires on Cu foam for remitting capacity fading of Li-ion battery anodes. Sci. Rep. 2018, 8, 6530. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Zhang, M.; Li, B.; Li, Y.; Wang, Z. Ag particles modified CuxO (x = 1, 2) nanowires on nanoporous Cu-Ag bimetal network for antibacterial applications. Mater. Lett. 2020, 258, 126823. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef] [Green Version]
- Poulopoulos, P.; Baskoutas, S.; Pappas, S.D.; Garoufalis, C.S.; Droulias, S.A.; Zamani, A.; Kapaklis, V. Intense quantum confinement effects in Cu2O thin films. J. Phys. Chem. C 2011, 115, 14839–14843. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Surya Prakash, G.K.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci. 2012, 5, 7833. [Google Scholar] [CrossRef]
- Earth System Research Laboratory. Global greenhouse gas reference network: Trends in atmospheric carbon dioxide. Available online: www.esrl.noaa.gov/gmd/ccgg/trends/mlo.html (accessed on 16 November 2020).
- Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report; Intergovernmental Panel on Climate Change: Incheon, Korea, 2014. [Google Scholar]
- Intergovernmental Panel on Climate Change. IPCC Special Report on the Impacts of Global Warming of 1.5 °C-Summary for Policy Makers; Intergovernmental Panel on Climate Change: Incheon, Korea, 2018. [Google Scholar]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89. [Google Scholar] [CrossRef]
- Montoya, J.H.; Shi, C.; Chan, K.; Nørskov, J.K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 2015, 6, 2032–2037. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solutions. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Murata, A.; Suzuki, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 1986, 15, 897–898. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Murata, A.; Takahashi, R.; Suzuki, S. Enhanced formation of ethylene and alcohols at ambient temperature and pressure in electrochemical reduction of carbon dioxide at a copper electrode. J. Chem. Soc. Chem. Commun. 1988, 0, 17. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: A classification problem. ChemPhysChem 2017, 18, 3266–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gameel, K.M.; Sharafaldin, I.M.; Abourayya, A.U.; Biby, A.H.; Allam, N.K. Unveiling CO adsorption on Cu surfaces: New insights from molecular orbital principles. Phys. Chem. Chem. Phys. 2018, 20, 25892–25900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hao, X.; Duan, T.; Wang, B. Adsorption and activation of CO and H2, the corresponding equilibrium phase diagrams under different temperature and partial pressures over Cu(100) surface: Insights into the effects of coverage and solvent effect. Fuel Process. Technol. 2017, 156, 253–264. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Loffler, M.; Khanipour, P.; Kulyk, N.; Mayrhofer, K.J.J.; Katsounaros, I. Insights into liquid product formation during carbon dioxide reduction on copper and oxide-derived copper from quantitative real-time measurements. ACS Catal. 2020, 10, 6735–6740. [Google Scholar] [CrossRef]
- Kibria, M.G.; Dinh, C.-T.; Seifitokaldani, A.; De Luna, P.; Burdyny, T.; Quintero-Bermudez, R.; Ross, M.B.; Bushuyev, O.S.; García de Arquer, F.P.; Yang, P.; et al. A surface reconstruction route to high productivity and selectivity in CO2 electroreduction toward C2+ hydrocarbons. Adv. Mater. 2018, 30, 1804867. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, J.; Zhang, B.; Tan, D.; Yao, L.; Zheng, L.; Zhang, F.; Liu, L.; Cheng, X.; Han, B. Boron-doped CuO nanobundles for electroreduction of carbon dioxide to ethylene. Green Chem. 2020, 22, 2750–2754. [Google Scholar] [CrossRef]
- Ting, L.R.; Pique, O.; Lim, S.Y.; Tanhaei, M.; Calle-Vallejo, F.; Yeo, B.S. Enhancing CO2 electroreduction to ethanol on copper−silver composites by opening an alternative catalytic pathway. ACS Catal. 2020, 10, 4059–4069. [Google Scholar] [CrossRef]
- Mandal, L.; Yang, K.R.; Motapothula, M.R.; Ren, D.; Lobaccaro, P.; Patra, A.; Sherburne, M.; Batista, V.S.; Yeo, B.S.; Ager, J.W.; et al. Investigating the role of copper oxide in electrochemical CO2 reduction in real time. ACS Appl. Mater. Interfaces 2018, 10, 8574–8584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Chen, C.; Yan, S.; Zhong, J.; Zhang, B.; Cheng, Z. Cu@Bi nanocone induced efficient reduction of CO2 to formate with high current densities. Appl. Catal. A 2020, 598, 117545. [Google Scholar] [CrossRef]
- Jeon, H.S.; Kunze, S.; Scholten, F.; Cuenya, B.R. Prism-shaped Cu nanocatalysts for electrochemical CO2 reduction to ethylene. ACS Catal. 2018, 8, 531–535. [Google Scholar] [CrossRef]
- Jeong, H.M.; Kwon, Y.; Won, J.H.; Lum, Y.; Cheng, M.-J.; Kim, K.H.; Head-Gordon, M.; Kang, J.K. Atomic-scale spacing between copper facets for the electrochemical reduction of carbon dioxide. Adv. Energy Mater. 2020, 10, 1903423. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, C.; Cerrillo, M.I.; Martinez, F.; Camarillo, R.; Rincon, J. Effect of carbon support on the catalytic activity of copper-based catalyst in CO2 electroreduction. Sep. Purif. Technol. 2020, 248, 117083. [Google Scholar] [CrossRef]
- Gao, D.; McCrum, I.T.; Deo, S.; Choi, Y.-W.; Scholten, F.; Wan, W.; Chen, J.G.; Janik, M.J.; Cuenya, B.R. Activity and selectivity control in CO2 electroreduction to multicarbon products over CuOx catalysts via electrolyte design. ACS Catal. 2018, 8, 10012–10020. [Google Scholar] [CrossRef]
- Wang, L. Electrochemical carbon monoxide reduction on polycrystalline copper: Effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 2018, 8, 7445–7454. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Verdaguer-Casadevall, A.; Li, C.W.; Johansson, T.P.; Scott, S.B.; McKeown, J.T.; Kumar, M.; Stephens, I.E.L.; Kanan, M.W.; Chorkendorff, I. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 2015, 137, 9808–9811. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, M.-H.; Huang, B. Cu2O nanoparticles with both {100} and {111} facets for enhancing the selectivity and activity of CO2 electroreduction to ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Xiao, H.; Goddard, W.A.; Cheng, T.; Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6685. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Cheng, T.; Goddard, W.A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111). J. Am. Chem. Soc. 2017, 139, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Jung, H.; Kim, N.-K.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorovic, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electroredeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Gupta, N.; Gattrell, M.; MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 2006, 36, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Handoko, A.-D.; Ong, C.W.; Huang, Y.; Lee, Z.G.; Lin, L.; Panetti, G.B.; Yeo, B.S. Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C 2016, 120, 20058–20067. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew. Chem. Int. Ed. Engl. 2013, 52, 2459–2462. [Google Scholar] [CrossRef]
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays. Angew. Chem. Int. Ed. 2016, 55, 6680–6684. [Google Scholar] [CrossRef]
- Fields, M.; Hong, X.; Nørskov, J.K.; Chan, K. Role of subsurface oxygen on Cu surfaces for CO2 electrochemical reduction. J. Phys. Chem. C 2018, 122, 16209–16215. [Google Scholar] [CrossRef]
- Favaro, M.; Xiao, H.; Cheng, T.; Goddard, W.A.; Yano, J.; Crumlin, E.J. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eilert, A.; Cavalca, F.; Roberts, F.S.; Osterwalder, J.; Liu, C.; Favaro, M.; Crumlin, E.J.; Ogasawara, H.; Friebel, D.; Pettersson, L.G.M.; et al. Subsurface oxygen in oxide-derived copper electrocatalysts for carbon dioxide reduction. J. Phys. Chem. Lett. 2017, 8, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cao, M.; Jiang, X.; Wang, M.; Shen, Y. A catalyst based on copper-cadium bimetal for electrochemical reduction of CO2 to CO with high faradaic efficiency. Electrochim. Acta 2018, 271, 511–550. [Google Scholar] [CrossRef]
- Lu, J.-J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.-J.; Jiao, F. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 2018, 30, 1803111. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly efficient electroreduction of CO2 to methanol on palladium–copper bimetallic aerogels. Angew. Chem. Int. Ed. 2018, 57, 14149–14153. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.J.; Kenis, P.A.J.; Gewirth, A.A. Nanoporous copper−silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Y.; Ren, D.; Handoko, A.D.; Seh, Z.W.; Hirunsit, P.; Yeo, B.S. On the role of sulfur for the selective electrochemical reduction of CO2 to formate on CuSx catalysts. ACS Appl. Mater. Interfaces 2018, 10, 28572–28581. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Yu, Y.; Tian, L.; Zhao, W.; Zhang, B. Cu2O nanocrystals: Surfactant-free room-temperature morphology-modulated synthesis and shape-dependent heterogeneous organic catalytic activities. J. Phys. Chem. C 2011, 115, 31, 15288–15296. [Google Scholar] [CrossRef]
- Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M.T.M.; Mul, G.; Baltrusaitis, J. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 2014, 16, 12194–12201. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective electroreduction of CO2 toward ethylene on nano dendritic copper catalysts at high current density. Adv. Energy Mater. 2017, 7, 1602114. [Google Scholar] [CrossRef]
- Li, J.; Kuang, Y.; Meng, Y.; Tian, X.; Hung, W.-H.; Zhang, X.; Li, A.; Xu, M.; Zhou, W.; Ku, H.-S.; et al. Electroreduction of CO2 to formate on a copper-based electrocatalyst at high pressures with high energy conversion efficiency. J. Am. Chem. Soc. 2020, 142, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Akhade, S.A.; Luo, W.; Nie, X.; Bernstein, N.J.; Asthagiri, A.; Janik, M.J. Poisoning effect of adsorbed CO during CO2 electroreduction on late transition metals. Phys. Chem. Chem. Phys. 2014, 16, 20429–20435. [Google Scholar] [CrossRef]
- Lum, Y.; Yue, B.; Lobaccaro, P.; Bell, A.T.; Ager, J.W. Optimizing C−C coupling on oxide-derived copper catalysts for electrochemical CO2 reduction. J. Phys. Chem. C 2017, 121, 14191–14203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Eng. Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Heli, H.; Jafarian, M.; Mahjani, M.G.; Gobal, F. Electro-oxidation of methanol in copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Panah, N.B.; Danaee, I.; Ghamsari, Z.G. Effect of electrochemical surface pretreatment on electro-catalytic activity of copper for ethanol oxidation. Surf. Eng. Appl. Electrochem. 2019, 55, 630–637. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Cho, M.; Seo, J.G. Electrochemical deposition of self-supported bifunctional copper oxide electrocatalyst for methanol oxidation and oxygen evolution reaction. J. Ind. Eng. Chem. 2019, 76, 515–523. [Google Scholar] [CrossRef]
- Anantharaj, S.; Sugime, H.; Noda, S. Ultrafast growth of Cu(OH)2–CuO nanoneedle array on Cu foil for methanol oxidation electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 27327–27338. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.M.; Pawar, B.S.; Inamdar, A.I.; Kim, J.; Jo, Y.; Cho, S.; Mali, S.S.; Hong, C.K.; Kwak, J.; Kim, H.; et al. In-situ synthesis of Cu(OH)2 and CuO nanowire electrocatalysts for methanol electro-oxidation. Matter. Lett. 2017, 187, 60–63. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Wang, A.L.; Li, G.R.; Wang, J.W.; Ou, Y.N.; Tong, Y.X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012, 22, 5656–5665. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, D.; Chen, D.; Waller, G.; Bu, Y.; Wang, Z.; Liu, M. Three-dimensional ultrathin Ni(OH)2 nanosheets grown on nickel foam for high performance supercapacitors. Nano Energy 2015, 11, 154–161. [Google Scholar] [CrossRef] [Green Version]
- El-Said, W.A.; AlMalki, W.A.; Sayed, E.M.; El-Hady, D.A.; Alshitari, W. Development of copper oxide nanostructures modified indium tin oxide electrode for electrochemical catalytically oxidation of methanol. Mater. Lett. 2020, 279, 128498. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Wang, K.; Du, G.; Asiri, A.M.; Sun, X. Cu(OH)2@CoCO3(OH)2·nH2O core–shell heterostructure nanowire array: An efficient 3D anodic Catalyst for oxygen evolution and methanol electrooxidation. Small 2017, 12, 1602755. [Google Scholar] [CrossRef]
- Torto, N.; Ruzgas, T.; Gorton, L. Electrochemical oxidation of mono- and disaccharides at fresh as well as oxidized copper electrodes in alkaline media. J. Electroanal. Chem. 1999, 464, 252–258. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kang, P.; Huang, H.-Q.; Liu, Z.-G.; Li, G.; Guo, Z. Porous CuO nanobelts assembly film for nonenzymatic electrochemical determination of glucose with high fabrication repeatability and sensing stability. Sens. Actuat. B 2020, 307, 127639. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Archetypal sandwich-structured CuO for high performance non-enzimatic sensing of glucose. Nanoscale 2013, 5, 2089. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Tarlochan, F. Highly efficient nonenzymatic glucose sensors based on CuO nanoparticles. Appl. Surf. Sci. 2019, 481, 712–722. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-dimensional copper foam supported CuO nanowire arrays: An efficient non-enzymatic glucose sensor. Electrochim. Acta 2017, 235, 519–526. [Google Scholar] [CrossRef]
- Li, C.; Su, Y.; Zhang, S.; Lv, X.; Xia, H.; Wang, Y. An improved sensitivity nonenzymatic glucose biosensor based on a CuxO modified electrode. Biosens. Bioelectron. 2010, 26, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-H.; Weng, W.-L.; Lee, C.-Y.; Liao, C.-N. Electrochemical cycling-induced spiky CuxO/Cu nanowire array for glucose sensing. ACS Omega 2019, 4, 12222–12229. [Google Scholar] [CrossRef] [Green Version]
- Babu, T.G.S.; Ramachandran, T. Development of highly sensitive non-enzymatic sensor for the selective determination of glucose and fabrication of a working model. Electrochim. Acta 2010, 55, 1612–1618. [Google Scholar] [CrossRef]
- Dat, P.V.; Viet, N.X. Facile synthesis of novel areca flower like Cu2O nanowire on copper foil for a highly sensitive enzyme-free glucose sensor. Mater. Sci. Eng. C 2019, 103, 109758. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Stanley, J.; Ramachandran, T.; Nair, B.G.; Babu, T.G.S. Cupric oxide modified screen printed electrode for the nonenzymatic glucose sensing. J. Nanosci. Nanotech. 2016, 16, 8772–8778. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Fu, J.; Wang, L.; Chen, S.; Li, P.; Li, H.; Song, Y. Electrolyte-controllable synthesis of CuxO with novel morphology and their application in glucose sensors. RSC Adv. 2014, 4, 52067. [Google Scholar] [CrossRef]
- Lu, C.; Li, Z.; Ren, L.; Su, N.; Lu, D.; Liu, Z. In situ oxidation of Cu2O crystal for electrochemical detection of glucose. Sensors 2019, 19, 2926. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fu, J.; Hou, H.; Song, Y. A facile strategy to prepare Cu2O/Cu electrode as a sensitive enzyme-free glucose sensor. Int. J. Electrochem. Sci. 2012, 7, 12587–12600. [Google Scholar]
- Xu, L.; Yang, Q.; Liu, X.; Liu, J.; Sun, X. One-dimensional copper oxide nanotube arrays: Biosensors for glucose detection. RSC Adv. 2014, 4, 1449. [Google Scholar] [CrossRef]
- Zhang, W.; Li, R.; Xing, L.; Wang, X.; Gou, X. Carnation-like CuO hierarchical nanostructures assembled by porous nanosheets for nonenzymatic glucose sensing. Electroanalysis 2016, 28, 2214–2221. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Karakocak, B.B.; Kavadiya, S.; Soundappan, T.; Biswas, P. A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens. Actuator. B 2018, 258, 745–752. [Google Scholar] [CrossRef]
- Jayasingha, L.; Jayathilaka, C.; Kumara, R.; Ohara, K.; Kaumal, M.; Guewardene, S.; Dissanayake, D.; Jayanetti, S. Nanoporous Cu2O nanotube/nanorod array electrodes for non-enzymatic glucose sensing with high sensitivity and very low detection limit. Electrochim. Acta 2020, 329, 125177. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Wang, Z.; Qin, C.; Zhang, M.; Li, Y. Porous CuxO/Ag2O (x = 1, 2) nanowires anodized on nanoporous Cu-Ag bimetal network as a self-supported flexible electrode for glucose sensing. Appl. Surf. Sci. 2020, 515, 146062. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Qin, C.; Wang, Z.; Zhao, W.; Li, Y. Flexible free-standing CuxO/Ag2O (x = 1, 2) nanowires integrated with nanoporous Cu-Ag network composite for glucose sensing. Nanomaterials 2020, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Zou, X.; Liu, Q.; Li, S.; Kang, C.; Xiang, W. A highly sensitive non-enzymatic glucose sensor based on CuS nanosheets modified Cu2O/CuO nanowire arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187. [Google Scholar] [CrossRef]
- Wei, H.; Sun, J.-J.; Guo, L.; Li, X.; Chen, G.-N. Highly enhanced electrocatalytic oxidation of glucose and shikimic acid at a disposable electrically heated oxide covered copper electrode. Chem. Commun. 2009, 2842. [Google Scholar] [CrossRef]
- Luo, M.Z.; Baldwin, R.P. Characterization of carbohydrate oxidation at copper electrodes. J. Electroanal. Chem. 1995, 387, 87. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Zhang, Q.; Qin, C.; Inoue, A.; Guo, W. Formation and evolution of ultrathin Cu2O nanowires on NPC ribbon by anodizing for photocatalytic degradation. Appl. Surf. Sci. 2020, 506, 144816. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, K. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dutta, B. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vernardou, D.; Dorsos, H.; Fasoulas, J.; Koudoumas, E.; Katsarakis, N. Photocatalytic properties of chemically grown vanadium oxide at 65 °C. Thin Solid Films 2014, 555, 169–172. [Google Scholar] [CrossRef]
- Zhou, T.W.; Zang, Z.G.; Wei, J.; Zheng, J.F.; Hao, J.Y.; Ling, F.L.; Tang, X.S.; Fang, L.; Zhou, M. Efficient charge carrier separation and excellent visible light photoresponse in Cu2O nanowires. Nano Energy 2018, 50, 118–125. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Mei, L.; Wang, X. Enhanced photocatalytic degradation of rhodamine B by Cu2O coated silicon nanowire arrays in presence of H2O2. J. Mater. Sci. Technol. 2014, 30, 1124–1129. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Zhang, Q.; Qin, C.; Zhao, W.; Wang, Z.; Inoue, A. Ultrafine Cu2O/CuO nanosheet arrays integrated with NPC/BMG composite rod for photocatalytic degradation. App. Surf. Sci. 2019, 483, 285–293. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, C.; Deng, Y.; Liu, L.; Wu, Y.; Hu, W. The manufacture of porous cuprous oxide film with photocatalytic properties via an electrochemical–chemical combination method. RSC Adv. 2013, 3, 6763. [Google Scholar] [CrossRef]
- Kulkarni, S.K. Nanothechnology: Principles and Practices, 3rd ed.; Springer: New York, NY, USA, 2015; p. 418. [Google Scholar]
- Grez, P.; Henríquez, R.; Muñoz, E.; Rojas, C.; Moreno, S.; Sessarego, G.; Heyser, C.; Celedón, C.; Schrebler, R. Sonoelectrochemical synthesis of nanostructured of p-Cu2O and n-Fe2O3 and their application for photoelectrochemical splitting of water. Inter. J. Electrochem. Sci. 2019, 14, 5646–5653. [Google Scholar] [CrossRef]
- Mirzaei, M.; Soleymani, A.P.; Ashrafi, A.; Momeni, M.M. Electrochemically enhanced hydrothermal production of cupric oxide photoelectrode on copper substrate. J. Electrochem. Soc. 2020, 167, 066507. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.S. Photoelectrochemical water splitting with p-type metal oxide semiconductor photocathodes. ChemSusChem 2019, 12, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-C.; Hou, S.; Huang, M.-H.; Li, Y.-B.; Li, T.; Xiao, F.-X. Electrochemically anodized one-dimensional semiconductors: A fruitful platform for solar energy conversion. J. Phys. Energy 2019, 1, 022002. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Li, S.; Zhang, B.; Wang, M.; Shen, Y. Carbon coated Cu2O nanowires for photo-electrochemical water splitting with enhanced activity. Appl. Surf. Sci. 2015, 358, 404–411. [Google Scholar] [CrossRef]
- Zimbovskii, D.S.; Baranov, A.N. Synthesis of Cu2O-based heterostructures and their photocatalytic properties for water splitting. Inorg. Mater. 2020, 56, 366–373. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef]
- John, S.; Vadla, S.S.; Roy, S.C. High photoelectrochemical activity of CuO nanoflakes grown on Cu foil. Electrochim. Acta 2019, 319, 390–399. [Google Scholar] [CrossRef]
- John, S.; Roy, S.C. CuO/Cu2O nanoflake/nanowire heterostructure photocathode with enhanced surface area for photoelectrochemical solar energy conversion. Appl. Surf. Sci. 2020, 509, 144703. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, Z.; Chen, H.; Ye, D.; Guo, L.; Fan, Z.; Yang, S. Current progress in developing metal oxide nanoarrays-based photoanodes for photoelectrochemical water splitting. Sci. Bull. 2019, 64, 1348–1380. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.S.; Kim, J.; Lee, M.J.; Son, Y.J.; Jeong, J.; Chung, D.Y.; Lim, A.; Choe, H.; Park, H.S.; Sung, Y.-E. Electrochemical synthesis of nanoporous tungsten carbide and its application as electrocatalysts for photoelectrochemical cells. Nanoscale 2017, 9, 5413–5424. [Google Scholar] [CrossRef]






| Possible CO2 Reduction Reactions | E0 [V vs. RHE] |
|---|---|
| CO2 + 2H+ + 2e− → HCOOH(aq) | –0.12 |
| CO2 + 4H+ + 4e− → C(s) + 2H2O | 0.21 |
| CO2 + 6H+ + 6e− → CH3OH(aq) + H2O | 0.03 |
| CO2 + 8H+ + 8e− → CH4(g) + 2H2O | 0.17 |
| 2CO2 + 10H+ + 10e− → CH3CHO(aq) + 3H2O | 0.06 |
| 2CO2 + 12H+ + 12e− → C2H4(g) + 4H2O | 0.08 |
| 2CO2 + 14H+ + 14e− → C2H6(g) + 4H2O | 0.14 |
| 3CO2 + 16H+ + 16e− → C2H5CHO(aq) + 5H2O | 0.09 |
| 3 CO2 + 18H+ + 18e− → C3H7HO(aq) + 5H2O | 0.10 |
| Morphology | Synthesis Method | Electrolyte | Potential [vs. RHE] | Faradaic Efficiency | Ref. | ||
|---|---|---|---|---|---|---|---|
| C2+ | C1 | Formate | |||||
| CuO nanoflowers | electrochemical pulsed oxidation | 0.1 M KHCO3 | −1.3 V | 9% | 4% | 30% | [58] |
| Cu2O nanoparticles | square-wave electrochemical redox treatment | 0.1 M KHCO3 45 atm. CO2 saturated | −0.64 V | - | - | 98% | [128] |
| CuOx nanoneedles | anodization | 0.1 M KHCO3 | −0.8 V | 43% | <1% | 2.4% | [93] |
| CuOx nanocrystals | electrodeposition | 0.1 M KHCO3 | −0.8 V | 38% | <1% | 12% | [93] |
| CuOx nanoparticles | thermal annealing | 0.1 M KHCO3 | −0.8 V | 21% | – | - | [93] |
| Cu foil | - | 0.1 M KHCO3 | −0.8 V | 4% | 47% | - | [93] |
| CuOx nanowires (collapsed after reduction) | anodization followed by rapid reduction (10 min at −4.0 V vs. Ag/AgCl) | 0.1 M KHCO3 | −1.05 V | 38% | <1% | - | [106] |
| CuOx nanowires (collapsed after reduction) | anodization followed by slow reduction (100 min at −1.15 V vs. Ag/AgCl) | 0.1 M KHCO3 | 1.05 V | 29% | 7% | - | [106] |
| CuOx nanowires | anodization | 0.1 M KHCO3 | −1.08 V | 38% | 1.3% | - | [106] |
| Cu foil | - | 0.1 M KHCO3 | −1.08 V | 15% | 24% | - | [106] |
| Material | Method of Synthesis | Morphology | Linear Response Range | Limit of Detection | Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| CuO@GC | Wet-chemical method | Nanowires with nanoflower particles | 1–850 µM | 0.25 µM | 2062 µA mM−1 cm−2 | [145] |
| CuO/CuOx/Cu | Electrooxidation: potentiodynamic | Rough and porous | Up to 15 mM | 0.05 µM | 1890 µA mM−1 cm−2 | [149] |
| Cu2O@Cu | Galvanostatic anodization and annealing under Ar flow | Flower-like nanowires with length > 5 µm | 1 µM–2 mM | 0.58 µM | 4060 µA mM−1 cm−2 | [150] |
| CuxO@Cu | Electrooxidation: potentiodynamic | Spike covered nanowire array | 10 µM–7 mM | 10 µM | 1210 µA mM−1 cm−2 | [148] |
| CuO @SPE | Electrooxidation: potentiodynamic | Flower-like nanoparticles | 0.5 µM–15 mM | 0.06 µM | 3225 µA mM−1 cm−2 | [151] |
| CuxO@Cu nanoparticles@ Cu foil | Thermal oxidation | Nanowire array | Up to 4.0 mM | 49 µM | 1620 µA mM−1 cm−2 | [147] |
| CuxO@Cu | Potentiostatic anodization | Microparticles 100–800 nm | 25 µM–9.05 mM | 14.3 µM | 452.4 µA mM−1 | [152] |
| CuO@ Cu foam | Hydrothermal and annealing in air | Nanowires and nanoflowers | 0.10 µM–0.50 mM | 0.02 µM | 32330 µA mM−1 cm−2 | [146] |
| Cu2O@ Cu foil | Galvanostatic polarization | Cubic nanoparticles 30–150 nm in diameter | 0.1–1 mM | 2.57 µM | 2524.9 µA mM−1 cm−2 | [153] |
| CuO | Wet-chemical method/microwave assisted | Microparticles with sandwich-like structure | Up to 3.2 mM | ~1 µM | 5342.8 µA mM−1 cm−2 | [144] |
| Cu2O@Cu foil | Potentiostatic polarization | Octahedron microcrystals | 0.05–6.75 mM | 37 µM | 62.29 µA mM−1 | [154] |
| CuO | Galvanostatic anodization | Nanotube array with 300 nm diameter and 15 µm length | 5 µM–3.0 mM | 0.1 µM | 1890 µA mM−1 cm−2 | [155] |
| CuO UPN | Wet-chemical method and annealing in air | Carnation-like particles 2.5 µm in diameter composed of 15 nm thick nanosheets | 3 µM–5.3 mM | 0.098 µM | 3150 µA mM−1 cm−2 | [156] |
| Cu/Cu2O/CuO | Aerosol furnace reactor assisted synthesis | Hollow spheres with diameter of 0.05–3 µm | 0.5 µM–30 mM | 0.39 µM | 8726 µA mM−1 cm−2 | [157] |
| Cu2O | Galvanostatic anodization and annealing | Porous nanotube or nanorod array with diameter of 40–70 nm | Up to 0.1 mM | 0.015 | 5792.7 µA mM−1 cm−2 | [158] |
| Photocathode | Method of Synthesis | Morphology | Maximum Photocurrent Density | LSV Experimental Condition | Irradiation Source | Ref. |
|---|---|---|---|---|---|---|
| CuO on Cu substrate (Eg = 1.50 eV) | Galvanostatic anodization, hydrothermal and annealing | Nanoneedles or nanoflakes | −4.6 mA cm−2 (at 0.05 V vs. RHE) | 0.5 M Na2SO4, pH = 7 Range: 0.0 V to 0.8 V SR: 10 mV s−1 | 300 W XL with AM 1.5 G filter | [182] |
| Cu2O@CuO on Cu substrate (Eg = 2.01 eV) | Galvanostatic anodization and annealing | Nanoflowers | −1.54 mA cm−2 (at −0.3 V vs. Ag|AgCl) | 0.5 M Na2SO4, pH = 6 Range: −0.5 V to 0.1 V | 300 W XL with AM 1.5 G filter | [67] |
| Cu2O on Cu substrate | Potentiostatic anodization | Sponge-like | −0.304 mA cm−2 (at −0.6 V vs. SHE) | 0.5 M Na2SO4, pH = 9.6 | 35 W XL with a UV cut-off filter (100 mW cm−2) | [175] |
| Cu2O/CuO on Cu/ITO substrate | Galvanostatic anodization | Vertically aligned nanosheets | −1.54 mA cm−2 (at 0 V vs. NHE) | 0.05 M Na2SO5, pH = 6.82 Range: 0.0 V to 0.6 V SR: 5 mV s−1 | 300 W XL with AM 1.5 G filter (100 mW cm−2) | [31] |
| C-coated Cu2O on Cu substrate (Eg = 2.08 eV) | Galvanostatic anodization and annealing | Nanowires with 200 nm diameter | −2.7 mA cm−2 (at 0 V vs. RHE) | 0.5 M Na2SO4, pH = 6.23 Range: 0.0 V to 0.6 V SR: 5 mV s−1 | 300 W XL with AM 1.5 G filter (100 mW cm−2) | [178] |
| AZO/TiO2/RuOx-covered Cu2O/CuO on Cu/FTO substrate (Eg ~ 2.0 eV) | Galvanostatic anodization and annealing | Nanowire array with diameters 100–300 nm and lengths of 3–5 µm | −10 mA cm−2 (at −0.3 V vs. RHE) | 0.5 M Na2SO4 + 0.1 M KH2PO4, pH = 5.0 Range: −0.3 V to 0.6 V SR: 10 mV s−1 | 450 W XL with AM 1.5 G filter (100 mW cm−2) | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giziński, D.; Brudzisz, A.; Santos, J.S.; Trivinho-Strixino, F.; Stępniowski, W.J.; Czujko, T. Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions. Catalysts 2020, 10, 1338. https://doi.org/10.3390/catal10111338
Giziński D, Brudzisz A, Santos JS, Trivinho-Strixino F, Stępniowski WJ, Czujko T. Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions. Catalysts. 2020; 10(11):1338. https://doi.org/10.3390/catal10111338
Chicago/Turabian StyleGiziński, Damian, Anna Brudzisz, Janaina S. Santos, Francisco Trivinho-Strixino, Wojciech J. Stępniowski, and Tomasz Czujko. 2020. "Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions" Catalysts 10, no. 11: 1338. https://doi.org/10.3390/catal10111338
APA StyleGiziński, D., Brudzisz, A., Santos, J. S., Trivinho-Strixino, F., Stępniowski, W. J., & Czujko, T. (2020). Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions. Catalysts, 10(11), 1338. https://doi.org/10.3390/catal10111338