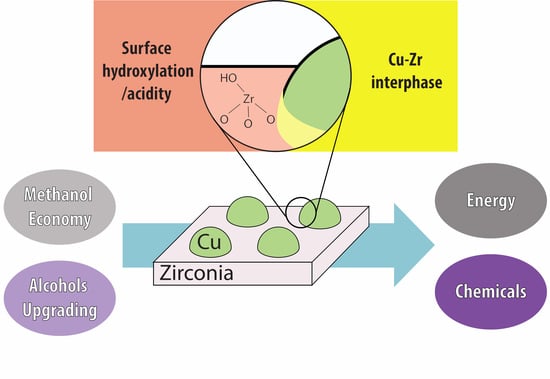
Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes
Abstract
:1. Introduction
2. Preparation and Properties of Copper–Zirconia Catalysts
3. Copper–Zirconia Catalysts for the Methanol Economy
4. Copper–Zirconia Catalysts for the Dehydrogenative Coupling Reaction
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.J.; Kang, D.C.; Pyen, S.H.; Shin, M.; Suh, Y.W.; Han, H.; Shin, C.H. Production of H2-free CO by decomposition of formic acid over ZrO2catalysts. Appl. Catal. A Gen. 2017, 531, 13–20. [Google Scholar] [CrossRef]
- Tada, S.; Katagiri, A.; Kiyota, K.; Honma, T.; Kamei, H.; Nariyuki, A.; Uchida, S.; Satokawa, S. Cu species incorporated into amorphous ZrO2 with high activity and selectivity in CO2-to-methanol hydrogenation. J. Phys. Chem. C 2018, 122, 5430–5442. [Google Scholar] [CrossRef]
- Ro, I.; Liu, Y.; Ball, M.R.; Jackson, D.H.K.; Chada, J.P.; Sener, C.; Kuech, T.F.; Madon, R.J.; Huber, G.W.; Dumesic, J.A. Role of the Cu-ZrO2 interfacial sites for conversion of ethanol to ethyl acetate and synthesis of methanol from CO2 and H2. ACS Catal. 2016, 6, 7040–7050. [Google Scholar] [CrossRef]
- Bossola, F.; Scotti, N.; Somodi, F.; Coduri, M.; Evangelisti, C.; Dal Santo, V. Electron-poor copper nanoparticles over amorphous zirconia-silica as all-in-one catalytic sites for the methanol steam reforming. Appl. Catal. B Environ. 2019, 258, 118016. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Evangelisti, C.; Psaro, R.; Ravasio, N. Dehydrogenative coupling promoted by copper catalysts: A way to optimise and upgrade bio-alcohols. Catal. Sci. Technol. 2017, 7, 1386–1393. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef] [PubMed]
- Zaccheria, F.; Scotti, N.; Ravasio, N. The role of copper in the upgrading of bioalcohols. ChemCatChem 2018, 10, 1526–1535. [Google Scholar] [CrossRef]
- Yuan, J.; Li, S.S.; Yu, L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen. Energy Environ. Sci. 2013, 6, 3308–3313. [Google Scholar] [CrossRef]
- Samson, K.; Sliwa, M.; Socha, R.P.; Góra-Marek, K.; Mucha, D.; Rutkowska-Zbik, D.; Paul, J.F.; Ruggiero-Mikoajczyk, M.; Grabowski, R.; Soczyński, J. Influence of ZrO2 structure and copper electronic state on activity of Cu/ZrO2 catalysts in methanol synthesis from CO2. ACS Catal. 2014, 4, 3730–3741. [Google Scholar] [CrossRef]
- Albuquerque, E.M.; Borges, L.E.P.; Fraga, M.A.; Sievers, C. Relationship between acid–base properties and the activity of ZrO2-based catalysts for the cannizzaro reaction of pyruvaldehyde to lactic acid. ChemCatChem 2017, 9, 2675–2683. [Google Scholar] [CrossRef]
- Aramend, M.A.; Bora, V.; Marinas, M.; Urbano, F.J. Synthesis and characterization of ZrO as an acid–base catalyst Dehydration–dehydrogenation of propan-2-ol. J. Chem. Soc. Faraday Trans. 1997, 93, 1431–1438. [Google Scholar] [CrossRef]
- Nagaiah, P.; Pramod, C.V.; Venkata Rao, M.; David Raju, B.; Rama Rao, K.S. Product selectivity as a function of ZrO2 phase in Cu/ZrO2 catalysts in the conversion of cyclohexanol. Catal. Lett. 2018, 148, 3042–3050. [Google Scholar] [CrossRef]
- Bonura, G.; Khassin, A.A.; Yurieva, T.M.; Cannilla, C.; Frusteri, F.; Frusteri, L. Structure control on kinetics of copper reduction in Zr–containing mixed oxides during catalytic hydrogenation of carbon oxides to methanol. Catal. Today 2018, 342, 39–45. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Wu, G.; Mao, D.; Lu, G. Influence of the component interaction over Cu/ ZrO2 catalysts induced with fractionated precipitation method on the catalytic performance for methanol steam reforming. RSC Adv. 2016, 6, 30176–30186. [Google Scholar] [CrossRef]
- Wu, Y.; Tan, L.; Zhang, T.; Xie, H.; Yang, G.; Tsubaki, N.; Chen, J. Effect of preparation method on ZrO2-based catalysts performance for isobutanol synthesis from syngas. Catalysts 2019, 9, 752. [Google Scholar] [CrossRef]
- Witoon, T.; Chalorngtham, J.; Dumrongbunditkul, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases. Chem. Eng. J. 2016, 293, 327–336. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Moroz, E.M.; Zyuzin, D.A.; Ishchenko, A.V.; Dolgikh, L.Y.; Strizhak, P.E. Structure of copper oxide species supported on monoclinic zirconia. J. Phys. Chem. C 2015, 119, 28828–28835. [Google Scholar] [CrossRef]
- Rhodes, M.D.; Bell, A.T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies. J. Catal. 2005, 233, 198–209. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Grabowski, R.; Słoczyński, J.; Śliwa, M.; Mucha, D.; Socha, R.P.; Lachowska, M.; Skrzypek, J. Influence of polymorphic ZrO2 phases and the silver electronic state on the activity of Ag/ZrO2 catalysts in the hydrogenation of CO2 to methanol. ACS Catal. 2011, 1, 266–278. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the role of low coordination sites in a cu metal nanoparticle: A step toward the selective synthesis of second generation biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the properties of supported copper oxide: Can the particle size induce acidic behaviour? Dalt. Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Teterycz, H.; Klimkiewicz, R.; Łaniecki, M. The role of Lewis acidic centers in stabilized zirconium dioxide. Appl. Catal. A Gen. 2003, 249, 313–326. [Google Scholar] [CrossRef]
- Xia, W.; Wang, F.; Wang, L.; Wang, J.; Chen, K. Highly selective lanthanum-modified zirconia catalyst for the conversion of ethanol to propylene: A combined experimental and simulation study. Catal. Lett. 2019, 150, 1–9. [Google Scholar] [CrossRef]
- Karapetrova, E.; Platzer, R.; Gardner, J.A.; Torne, E.; Sommers, J.A.; Evenson, W.E. Oxygen vacancies in pure tetragonal zirconia powders: Dependence on the presence of chlorine during processing. J. Am. Ceram. Soc. 2001, 84, 65–70. [Google Scholar] [CrossRef]
- Białas, A.; Kondratowicz, T.; Drozdek, M.; Kuśtrowski, P. Catalytic combustion of toluene over copper oxide deposited on two types of yttria-stabilized zirconia. Catal. Today 2015, 257, 144–149. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active sites and structure-activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Zhao, Q.; Shih, W.H.; Chang, H.L.; Andersen, P. The effect of curing on the thermal stability of Si-doped ZrO2 powders. Appl. Catal. A Gen. 2004, 262, 215–221. [Google Scholar] [CrossRef]
- Mayr, L.; Klötzer, B.; Zemlyanov, D.; Penner, S. Steering of methanol reforming selectivity by zirconia-copper interaction. J. Catal. 2015, 321, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Lam, E.; Larmier, K.; Wolf, P.; Tada, S.; Safonova, O.V.; Copéret, C. Isolated Zr surface sites on silica promote hydrogenation of CO2 to CH3OH in supported Cu catalysts. J. Am. Chem. Soc. 2018, 140, 10530–10535. [Google Scholar] [CrossRef]
- Ishikawa, S.; Jones, D.R.; Iqbal, S.; Reece, C.; Morgan, D.J.; Willock, D.J.; Miedziak, P.J.; Bartley, J.K.; Edwards, J.K.; Murayama, T.; et al. Identification of the catalytically active component of Cu-Zr-O catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2017, 19, 225–236. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P.; Aronne, A. Highly dispersed sol-gel synthesized Cu-ZrO2 materials as catalysts for oxidative steam reforming of methanol. Appl. Catal. A Gen. 2010, 372, 48–57. [Google Scholar] [CrossRef]
- Scotti, N.; Monticelli, D.; Zaccheria, F. Dispersed copper oxide: A multifaceted tool in catalysis. Inorganica Chim. Acta 2012, 380, 194–200. [Google Scholar] [CrossRef]
- Wang, L.C.; Liu, Q.; Chen, M.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C 2007, 111, 16549–16557. [Google Scholar] [CrossRef]
- Aguila, G.; Guerrero, S.; Baeza, P.; Araya, P. Study of the influence of the Cu/Ce loading ratio in the formation of highly active species on ZrO2 supported copper-ceria catalysts. Mater. Chem. Phys. 2019, 223, 666–675. [Google Scholar] [CrossRef]
- Huber, F.; Yu, Z.; Walmsley, J.C.; Chen, D.; Venvik, H.J.; Holmen, A. Nanocrystalline Cu-Ce-Zr mixed oxide catalysts for water-gas shift: Carbon nanofibers as dispersing agent for the mixed oxide particles. Appl. Catal. B Environ. 2007, 71, 7–15. [Google Scholar] [CrossRef]
- Liang, Q.; Wu, X.; Weng, D.; Lu, Z. Selective oxidation of soot over Cu doped ceria/ceria-zirconia catalysts. Catal. Commun. 2008, 9, 202–206. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Wu, D. Ternary copper-cerium-zirconium mixed metal oxide catalyst for direct CO2 hydrogenation to methanol. Mater. Chem. Phys. 2018, 219, 263–272. [Google Scholar] [CrossRef]
- Miura, H.; Nakahara, K.; Kitajima, T.; Shishido, T. Concerted functions of surface acid-base pairs and supported copper catalysts for dehydrogenative synthesis of esters from primary alcohols. ACS Omega 2017, 2, 6167–6173. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Barbosa, F.G.; Letichevsky, S.; Appel, L.G. The one-pot ethyl acetate syntheses: The role of the support in the oxidative and the dehydrogenative routes. Appl. Catal. A Gen. 2010, 380, 113–117. [Google Scholar] [CrossRef]
- Larmier, K.; Liao, W.C.; Tada, S.; Lam, E.; Verel, R.; Bansode, A.; Urakawa, A.; Comas-Vives, A.; Copéret, C. CO2-to-methanol hydrogenation on zirconia-supported copper nanoparticles: Reaction intermediates and the role of the metal–support interface. Angew. Chem. Int. Ed. 2017, 56, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A comparative discussion of the catalytic activity and CO2-selectivity of Cu-Zr and Pd-Zr (intermetallic) compounds in methanol steam reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, I.; Douthwaite, M.; Iqbal, S.; Hayward, J.S.; Davies, T.E.; Bartley, J.K.; Miedziak, P.J.; Hirayama, J.; Morgan, D.J.; Willock, D.J.; et al. The hydrogenation of levulinic acid to Γ-valerolactone over Cu–ZrO2 catalysts prepared by a pH-gradient methodology. J. Energy Chem. 2019, 36, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Fan, G.; Yang, L.; Li, F. Highly efficient transformation of Γ-valerolactone to valerate esters over structure-controlled copper/zirconia catalysts prepared via a reduction-oxidation route. Appl. Catal. A Gen. 2017, 543, 180–188. [Google Scholar] [CrossRef]
- Tsai, A.P.; Kameoka, S.; Nozawa, K.; Shimoda, M.; Ishii, Y. Intermetallic: A pseudoelement for catalysis. Acc. Chem. Res. 2017, 50, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shuai, K.; Xu, B. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Carraro, F.; Fapohunda, A.; Paganini, M.C.; Agnoli, S. Morphology and size effect of ceria nanostructures on the catalytic performances of Pd/CeO2 catalysts for methanol decomposition to syngas. ACS Appl. Nano Mater. 2018, 1, 1492–1501. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I.; Du, S.; Wood, J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming. Fuel 2018, 232, 672–683. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdullah, A.Z.; Mohamed, A.R. Recent development in catalytic technologies for methanol synthesis from renewable sources: A critical review. Renew. Sustain. Energy Rev. 2015, 44, 508–518. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 2019, 34, 20–33. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.G. CO2 Hydrogenation to methanol over ZrO2-containing catalysts: Insights into ZrO2 induced synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Martínez-Arias, A. Catalytic hydrogen production through WGS or steam reforming of alcohols over Cu, Ni and Co catalysts. Appl. Catal. A Gen. 2016, 518, 2–17. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Genova, I.; Dimitrov, M.; Sarcadi-Priboczki, E.; Venezia, A.M.; Kovacheva, D.; Scotti, N.; dal Santo, V. Nanostructured copper-zirconia composites as catalysts for methanol decomposition. Appl. Catal. B Environ. 2015, 165, 599–610. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.; White, M.G. Theoretical perspective of alcohol decomposition and synthesis from CO2 hydrogenation. Surf. Sci. Rep. 2013, 68, 233–272. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J. Water gas shift catalysis. Catal. Rev. Sci. Eng. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the mechanism of low-temperature water gas shift reaction on copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. A mechanistic study of methanol decomposition over Cu/SiO2, ZrO2/SiO2, and Cu/ZrO2/SiO2. J. Catal. 1999, 184, 357–376. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In-situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1997, 172, 222–237. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; De Jong, K.P.; De Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. Stabilization of Cu/ZnO/ZrO2 catalyst for methanol steam reforming to hydrogen by coprecipitation on zirconia support. J. Power Sources 2013, 238, 109–116. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishibe, H. High temperature steam reforming of methanol over Cu/ZnO/ZrO2 catalysts. Appl. Catal. B Environ. 2009, 91, 524–532. [Google Scholar] [CrossRef]
- Morterra, C.; Giamello, E.; Orio, L.; Volante, M. Formation and reactivity of Zr3+ centers at the surface of vacuum-activated monoclinic zirconia. J. Phys. Chem. 1990, 94, 3111–3116. [Google Scholar] [CrossRef]
- Jung, K.D.; Bell, A.T. Role of hydrogen spillover in methanol synthesis over Cu/ZrO2. J. Catal. 2000, 193, 207–223. [Google Scholar] [CrossRef]
- Ruano, D.; Cored, J.; Azenha, C.; Pérez-Dieste, V.; Mendes, A.; Mateos-Pedrero, C.; Concepción, P. Dynamic structure and subsurface oxygen formation of a working copper catalyst under methanol steam reforming conditions: An in situ time-resolved spectroscopic study. ACS Catal. 2019, 9, 2922–2930. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef]
- Stefanovich, E.V.; Shluger, A.L.; Catlow, C.R.A. Theoretical study of the stabilization of cubic-phase ZrO2 by impurities. Phys. Rev. B 1994, 49, 11560–11571. [Google Scholar] [CrossRef]
- Yao, C.Z.; Wang, L.C.; Liu, Y.M.; Wu, G.S.; Cao, Y.; Dai, W.L.; He, H.Y.; Fan, K.N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Pokrovski, K.A.; Rhodes, M.D.; Bell, A.T. Effects of cerium incorporation into zirconia on the activity of Cu/ZrO2 for methanol synthesis via CO hydrogenation. J. Catal. 2005, 235, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Bonura, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Catizzone, E.; Giordano, G.; Frusteri, F. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J. CO2 Util. 2018, 24, 398–406. [Google Scholar] [CrossRef]
- Tada, S.; Satokawa, S. Effect of Ag loading on CO2-to-methanol hydrogenation over Ag/CuO/ZrO2. Catal. Commun. 2018, 113, 41–45. [Google Scholar] [CrossRef]
- Tada, S.; Watanabe, F.; Kiyota, K.; Shimoda, N.; Hayashi, R.; Takahashi, M.; Nariyuki, A.; Igarashi, A.; Satokawa, S. Ag addition to CuO-ZrO2 catalysts promotes methanol synthesis via CO2 hydrogenation. J. Catal. 2017, 351, 107–118. [Google Scholar] [CrossRef]
- Oguchi, H.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Cu2O as active species in the steam reforming of methanol by CuO/ZrO2 catalysts. Appl. Catal. A Gen. 2005, 293, 64–70. [Google Scholar] [CrossRef]
- Mastalir, A.; Frank, B.; Szizybalski, A.; Soerijanto, H.; Deshpande, A.; Niederberger, M.; Schomäcker, R.; Schlögl, R.; Ressler, T. Steam reforming of methanol over Cu/ZrO2/CeO2 catalysts: A kinetic study. J. Catal. 2005, 230, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Grabow, L.C.; Mavrikakis, M. Mechanism of methanol synthesis on cu through CO2 and CO hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Tada, S.; Kayamori, S.; Honma, T.; Kamei, H.; Nariyuki, A.; Kon, K.; Toyao, T.; Shimizu, K.I.; Satokawa, S. Design of interfacial sites between Cu and amorphous ZrO2 dedicated to CO2-to-methanol hydrogenation. ACS Catal. 2018, 8, 7809–7819. [Google Scholar] [CrossRef]
- Lam, E.; Larmier, K.; Tada, S.; Wolf, P.; Safonova, O.V.; Copéret, C. Zr(IV) surface sites determine CH3OH formation rate on Cu/ZrO2/SiO2—CO2 hydrogenation catalysts. Chin. J. Catal. 2019, 40, 1741–1748. [Google Scholar] [CrossRef]
- Wu, G.; Sun, Y.; Li, Y.W.; Jiao, H.; Xiang, H.W.; Xu, Y. The nature of Cu/ZrO2 catalyst: Experimental and theoretical studies. J. Mol. Struct. THEOCHEM 2003, 626, 287–293. [Google Scholar] [CrossRef]
- Tang, Q.L.; Liu, Z.P. Identification of the active Cu phase in the water-gas shift reaction over Cu/ZrO2 from first principles. J. Phys. Chem. C 2010, 114, 8423–8430. [Google Scholar] [CrossRef]
- Polierer, S.; Jelic, J.; Pitter, S.; Studt, F. On the reactivity of the Cu/ZrO2 system for the hydrogenation of CO2 to methanol: A density functional theory study. J. Phys. Chem. C 2019, 123, 26904–26911. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.L.; Hong, Q.J.; Liu, Z.P. CO2 fixation into methanol at Cu/ZrO2 interface from first principles kinetic Monte Carlo. J. Catal. 2009, 263, 114–122. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Tada, S.; Larmier, K.; Büchel, R.; Copéret, C. Methanol synthesis: Via CO2 hydrogenation over CuO-ZrO2 prepared by two-nozzle flame spray pyrolysis. Catal. Sci. Technol. 2018, 8, 2056–2060. [Google Scholar] [CrossRef]
- Lin, S.; Xie, D.; Guo, H. Methyl formate pathway in methanol steam reforming on copper: Density functional calculations. ACS Catal. 2011, 1, 1263–1271. [Google Scholar] [CrossRef]
- Frank, B.; Jentoft, F.C.; Soerijanto, H.; Kröhnert, J.; Schlögl, R.; Schomäcker, R. Steam reforming of methanol over copper-containing catalysts: Influence of support material on microkinetics. J. Catal. 2007, 246, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Liao, P.H.; Yang, H.M. Preparation of catalyst Ni-Cu/CNTs by chemical reduction with formaldehyde for steam reforming of methanol. Catal. Lett. 2008, 121, 274–282. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Mateos-pedrero, C.; Boaventura, M.; Sousa, J.; Mendes, A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model. Appl. Catal. B Environ. 2018, 221, 371–379. [Google Scholar] [CrossRef]
- Azenha, C.S.R.; Mateos-Pedrero, C.; Queirós, S.; Concepción, P.; Mendes, A. Innovative ZrO2-supported CuPd catalysts for the selective production of hydrogen from methanol steam reforming. Appl. Catal. B Environ. 2017, 203, 400–407. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Homogeneous precipitation synthesis of CuO-ZrO2-CeO2-Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming for fuel cell applications. Energy Convers. Manag. 2014, 87, 928–937. [Google Scholar] [CrossRef]
- Das, D.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Gayen, A. Methanol steam reforming behavior of copper impregnated over CeO2-ZrO2 derived from a surfactant assisted coprecipitation route. Int. J. Hydrogen Energy 2015, 40, 10463–10479. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, V.G.; Abrokwah, R.Y.; Kuila, D. Synthesis of stable Cu-MCM-41 nanocatalysts for H2 production with high selectivity via steam reforming of methanol. Int. J. Hydrogen Energy 2015, 40, 10439–10452. [Google Scholar] [CrossRef] [Green Version]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MХZr1-XO2-Δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 198–207. [Google Scholar] [CrossRef]
- Ritzkopf, I.; Vukojević, S.; Weidenthaler, C.; Grunwaldt, J.D.; Schüth, F. Decreased CO production in methanol steam reforming over Cu/ZrO2 catalysts prepared by the microemulsion technique. Appl. Catal. A Gen. 2006, 302, 215–223. [Google Scholar] [CrossRef]
- Wu, G.S.; Mao, D.S.; Lu, G.Z.; Cao, Y.; Fan, K.N. The role of the promoters in Cu based catalysts for methanol steam reforming. Catal. Lett. 2009, 130, 177–184. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Bankar, S.B.; Survase, S.A.; Ojamo, H.; Granström, T. Biobutanol: The outlook of an academic and industrialist. RSC Adv. 2013, 3, 24734–24757. [Google Scholar] [CrossRef] [Green Version]
- Rajesh Kumar, B.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Moromi, S.K.; Hakim Siddiki, S.M.A.; Ali, M.A.; Kon, K.; Shimizu, K.I. Acceptorless dehydrogenative coupling of primary alcohols to esters by heterogeneous Pt catalysts. Catal. Sci. Technol. 2014, 4, 3631–3635. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Trivelli, X.; Capet, F.; Swesi, Y.; Favre-Réguillon, A.; Vanoye, L.; Dumeignil, F.; Gauvin, R.M. Deeper mechanistic insight into Ru pincer-mediated acceptorless dehydrogenative coupling of alcohols: Exchanges, intermediates, and deactivation species. ACS Catal. 2018, 8, 4719–4734. [Google Scholar] [CrossRef]
- Petrolini, D.D.; Cassinelli, W.H.; Pereira, C.A.; Urquieta-González, E.A.; Santilli, C.V.; Martins, L. Ethanol dehydrogenative reactions catalyzed by copper supported on porous Al-Mg mixed oxides. RSC Adv. 2019, 9, 3294–3302. [Google Scholar] [CrossRef] [Green Version]
- Inui, K.; Kurabayashi, T.; Sato, S. Direct synthesis of ethyl acetate from ethanol carried out under pressure. J. Catal. 2002, 212, 207–215. [Google Scholar] [CrossRef]
- Inui, K.; Kurabayashi, T.; Sato, S.; Ichikawa, N. Effective formation of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. J. Mol. Catal. A Chem. 2004, 216, 147–156. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; De Freitas, I.C.; Longo, E.; Bueno, J.M.C. Site-selective ethanol conversion over supported copper catalysts. Catal. Commun. 2012, 26, 122–126. [Google Scholar] [CrossRef]
- Liu, X.; Bai, S.; Zhuang, H.; Yan, Z. Preparation of Cu/ZrO2 catalysts for methanol synthesis from CO2/H2. Front. Chem. Sci. Eng. 2012, 6, 47–52. [Google Scholar] [CrossRef]
- Basahel, S.; Mokhtar, M.; Alsharaeh, E.; Ali, T.; Mahmoud, H.; Narasimharao, K. Physico-chemical and catalytic properties of mesoporous CuO-ZrO2 catalysts. Catalysts 2016, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO supported on tetragonal ZrO2 catalysts for N2O decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.; He, D.; Zhang, Q.; Wu, X.; Liang, Y.; Zhu, Q. Surface active structure of ultra-fine Cu/ZrO2 catalysts used for the CO2 + H2 to methanol reaction. Appl. Catal. A Gen. 2001, 218, 113–119. [Google Scholar] [CrossRef]
- Breen, J.P.; Ross, J.R.H. Methanol reforming for fuel-cell applications: Development of zirconia-containing Cu-Zn-Al catalysts. Catal. Today 1999, 51, 521–533. [Google Scholar] [CrossRef]
- Lee, J.F.; Zheng, F.S.; Chang, J.R. Structural investigation of solid-acid-promoted Pd/SDB catalysts for ethyl acetate production from ethanol. J. Phys. Chem. B 2002, 105, 3400–3404. [Google Scholar] [CrossRef]
- Hayashi, T.; Inagaki, T.; Itayama, N.; Baba, H. Selective oxidation of alcohol over supported gold catalysts: Methyl glycolate formation from ethylene glycol and methanol. Catal. Today 2006, 117, 210–213. [Google Scholar] [CrossRef]
- Sánchez, A.B.; Homs, N.; Fierro, J.L.G.; De La Piscina, P.R. New supported Pd catalysts for the direct transformation of ethanol to ethyl acetate under medium pressure conditions. Catal. Today 2005, 107–108, 431–435. [Google Scholar] [CrossRef]
- Hakim Siddiki, S.M.A.; Toyao, T.; Shimizu, K.I. Acceptorless dehydrogenative coupling reactions with alcohols over heterogeneous catalysts. Green Chem. 2018, 20, 2933–2952. [Google Scholar] [CrossRef]
- Ichikawa, N.; Sato, S.; Takahashi, R.; Sodesawa, T.; Inui, K. Dehydrogenative cyclization of 1,4-butanediol over copper-based catalyst. J. Mol. Catal. A Chem. 2004, 212, 197–203. [Google Scholar] [CrossRef]
- Inui, K.; Kurabayashi, T.; Sato, S. Direct synthesis of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. Appl. Catal. A Gen. 2002, 237, 53–61. [Google Scholar] [CrossRef]
- Balla, P.; Perupogu, V.; Vanama, P.K.; Komandur, V.R.C. Hydrogenation of biomass-derived levulinic acid to γ-valerolactone over copper catalysts supported on ZrO2. J. Chem. Technol. Biotechnol. 2016, 91, 769–776. [Google Scholar] [CrossRef]
- Du, X.L.; Bi, Q.Y.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Tunable copper-catalyzed chemoselective hydrogenolysis of biomass-derived γ-valerolactone into 1,4-pentanediol or 2-methyltetrahydrofuran. Green Chem. 2012, 14, 935–939. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, G.; Yang, L.; Li, F. Efficient conversion of furfural into cyclopentanone over high performing and stable Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2018, 561, 117–126. [Google Scholar] [CrossRef]
- Jones, D.R.; Iqbal, S.; Ishikawa, S.; Reece, C.; Thomas, L.M.; Miedziak, P.J.; Morgan, D.J.; Edwards, J.K.; Bartley, J.K.; Willock, D.J.; et al. The conversion of levulinic acid into γ-valerolactone using Cu-ZrO2 catalysts. Catal. Sci. Technol. 2016, 6, 6022–6030. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, X.; Zou, W.; Yue, H.; Tian, G.; Feng, S. Transfer hydrogenation of methyl levulinate into gamma-valerolactone, 1,4-pentanediol, and 1-pentanol over Cu-ZrO2 catalyst under solvothermal conditions. Catal. Commun. 2016, 76, 50–53. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Gonell, F.; Boronat, M.; Corma, A. Structure-reactivity relationship in isolated Zr sites present in Zr-zeolite and ZrO2 for the Meerwein-Ponndorf-Verley reaction. Catal. Sci. Technol. 2017, 7, 2865–2873. [Google Scholar] [CrossRef] [Green Version]
- Harianawala, H.; Kheur, M.; Bal, A. Biocompatibility of zirconia. J. Adv. Med. Dent. Sci. Res. 2016, 4, 35–39. [Google Scholar]
- Zhu, N.; Zhang, A.; Wang, Q.; He, P.; Fang, Y. Electrochemical detection of DNA hybridization using methylene blue and electro-deposited zirconia thin films on gold electrodes. Anal. Chim. Acta 2004, 510, 163–168. [Google Scholar] [CrossRef]









| Catalyst | Reaction | Preparation Procedure | Reaction Conditions | Catalytic Performances | Ref. |
|---|---|---|---|---|---|
| Cu/ZrO2 (80 wt % Cu) | MSR | Co-precipitation | S/C = 1.5; T = 250 °C | Prod. H2 = 400 mmol gcat−1 h−1 Conv. = -; Sel. CO < 0.1% | [78] |
| Cu/(t- and m-)ZrO2 (13 wt % Cu) | MSR | Microemulsion technique | S/C = 1.0; T = 250 °C | Prod. H2 = -; Conv. = 57%; CO = 0.02 vol.% | [99] |
| Cu/ZrO2 (Cu/Zr = 4 mol/mol) | MSR | Oxalate gel co-precipitation | S/C = 1.3; T = 260 °C | Prod. H2 = 350 mmol gcat−1 h−1 Conv. = 100%; CO = trace amount | [71] |
| Cu/ZrO2/CeO2 (15 mol % CuO) | MSR | Co-precipitation | S/C = 1.0; T = 220 °C | Prod. H2 = 0.38 mmol gcat−1 h−1 Conv. = 40%; CO = - | [91] |
| Cu/t-ZrO2 (10 wt % Cu) | MS | Complexation with citric acid | P = 80 bar; T = 250 °C | Prod. MeOH = 632 mmol gcat−1 h−1 Conv. = 15%; Sel. CO = 14% | [9] |
| Cu1Zr0.5/SiO2 (6.11 wt % Cu) | MS | Control Surface Reaction (CSR) | P = 30 bar; T = 250 °C | Prod. MeOH = 4 mmol gcat−1 h−1 Conv. = -; | [3] |
| Cu/a-Zr0.9@SiO2 (4.11 wt % Cu) | MS | Grafting | P = 25 bar; T = 230 °C | Prod. MeOH = 131 mmol gcat−1 h−1 Conv. = -%; Sel. CO = -% | [30] |
| Cu/a-ZrO2 (8 wt % Cu) | MS | Incipient wetness | P = 10 bar; T = 230 °C | Prod. MeOH = 1.4 mmol gcat−1 h−1 Conv. = -%; Sel. CO = -% | [81] |
| Cu/a-ZrO2-SiO2 (13.7 wt % Cu) | MSR | Incipient wetness | S/C = 1.3; T = 260 °C | Prod. H2 = 370 mmol gcat−1 h−1 Conv. = 73%; CO = trace amount | [4] |
| Catalyst | Substrate | Condition | T (°C) | C (%) | S (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu–Zn–Zr–Al–O | Ethanol | Gas phase | 240 | 82 | 76 | [108] |
| Cu/m-ZrO2 | Ethanol | Gas phase | 300 | 49 | 81 | [19] |
| Cu/m-ZrO2 | Ethanol | Gas phase | 200 | 45 | 73 | [100] |
| Cu/ZrO2/Al2O3 | Ethanol | Gas phase | 260 | 86 | 71 | [121] |
| Cu1Zr0.25/SiO2 | Ethanol | Gas phase | 200 | 30 | 33 | [3] |
| Cu-Zn-Zr-Al-O | Ethanol | Gas phase | 220 | 66 | 85 | [99] |
| Cu/a-ZrO2 | Ethanol | Batch | 250 | 89 | 98 | [5] |
| Cu/a-ZrO2 | 1-butanol | Batch | 250 | 98 | 100 | [5] |
| Cu/m-ZrO2 | 1-octanol | Batch | 170 | 99 | 68 | [39] |
| Cu–Zn–Zr–Al–O | 1,4-butanediol | Gas phase | 240 | 84 | 98 | [110] |
| Reaction | Focus | Significant Advances | Year | Ref. |
|---|---|---|---|---|
| DHC | Deep study of the reaction products and intermediates | Strong evidences of the hemiacetal mechanism as the active one | 2002 | [108] |
| MSR | Study on the actual active phase | Evidences of Cu+ role in promoting the reaction | 2005 | [78] |
| MSR | Study on the actual active phase | Insights on the cooperative action of Cu0 and Cu+ sites | 2006 | [99] |
| MSR | Correlation between exposed Cu phase and activity | No linear correlation found | 2006 | [71] |
| MSR | Study on the reaction mechanism | Two distinct catalytic sites: one for dehydrogenation and one for molecular hydrogen formation | 2007 | [91] |
| DHC | Use of mechanical mixture of different catalysts | Awareness on the role of the basic site | 2010 | [40] |
| MS | Impact of the crystal phases on the activity | Oxygen vacancies formation on t-ZrO2 result in abundant Lewis acid sites in the form of Cu+ sites | 2014 | [9] |
| MSR | Role of Cu-Zr interphase | Evidences on the role of Cu/ZrOxH phase boundaries on the activity | 2015 | [29] |
| DHC/MS | Synthesis of well-defined Cu-Zr catalysts. | Further emphasis on the Cu–Zr interphase for the activity | 2016 | [3] |
| DHC | Use of high SA zirconia and the preparation a catalyst with high Cu-Zr interdisperion | Elucidation on the cooperative role between Zr and Cu in promoting the reaction | 2017 | [5] |
| DHC | Comparison between Cu-based catalysts with different acid–base properties | Strong correlation between acid–base pairs and activity | 2017 | [39] |
| MD | Elucidation of Cu0 and Cu+ roles in methanol dehydrogenation | Cu+ sites responsible for the final CO formation from the intermediates on Cu0 sites, on which cleavage of the C-H and O-H bonds occur | 2018 | [69] |
| MS | Role of Zr sites | cus Zr (IV) sites act as Lewis acids and assist the CO2 activation Oxygen vacancies no longer critical | 2018 | [30] |
| MS | Maximization of the Cu-Zr interphase | CuxZryOz phase maximizes the Cu-Zr interaction and is favoured on a-ZrO2 | 2018 | [2,81] |
| MSR | Maximization of Cu active phase | Electron-poor metallic Cu nanoparticles act as all-in-one active site | 2019 | [4] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, N.; Bossola, F.; Zaccheria, F.; Ravasio, N. Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes. Catalysts 2020, 10, 168. https://doi.org/10.3390/catal10020168
Scotti N, Bossola F, Zaccheria F, Ravasio N. Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes. Catalysts. 2020; 10(2):168. https://doi.org/10.3390/catal10020168
Chicago/Turabian StyleScotti, Nicola, Filippo Bossola, Federica Zaccheria, and Nicoletta Ravasio. 2020. "Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes" Catalysts 10, no. 2: 168. https://doi.org/10.3390/catal10020168
APA StyleScotti, N., Bossola, F., Zaccheria, F., & Ravasio, N. (2020). Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes. Catalysts, 10(2), 168. https://doi.org/10.3390/catal10020168