CuZnZr-Zeolite Hybrid Grains for DME Synthesis: New Evidence on the Role of Metal-Acidic Features on the Methanol Conversion Step
Abstract
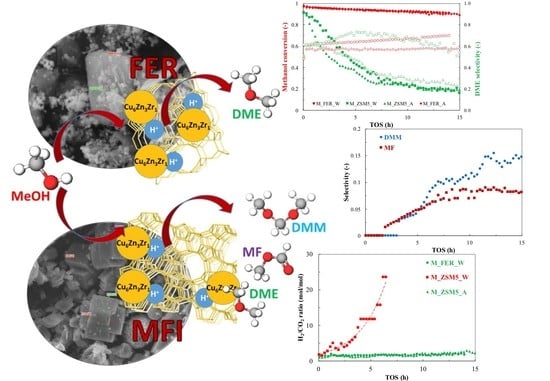
:1. Introduction
2. Results
2.1. Samples Preparation and Nomenclature
2.2. Structural Analysis
3. Discussion
3.1. Catalytic Test
3.2. Duration Test
4. Materials and Methods
4.1. Physicochemical Characterization
4.2. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fleisch, T.; Basu, A.; Sills, R. Introduction and advancement of a new clean global fuel: The status of DME developments in China and beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107. [Google Scholar] [CrossRef]
- Fleisch, T.; McCarthy, C.; Basu, A.; Udovich, C.; Charbonneau, P.; Slodowske, W.; Mikkelsen, S.-E.; McCandless, J. A New Clean Diesel Technology: Demonstration of ULEV Emissions on a Navistar Diesel Engine Fueled with Dimethyl Ether. SAE Tech. Pap. Ser. 1995. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Purita, A.; Giordano, G. Ferrierite vs. γ-Al2O3: The superiority of zeolites in terms of water-resistance in vapour-phase dehydration of methanol to dimethyl ether. J. Energy Chem. 2019, 30, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Bonura, G.; Cordaro, M.; Spadaro, L.; Cannilla, C.; Arena, F.; Frusteri, F. Hybrid Cu-ZnO-ZrO2/H-ZSM5 system for the direct synthesis of DME by CO2 hydrogenation. Appl. Catal. B Environ. 2013, 140, 16–24. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Pino, F.; Arrigo, R.; Giordano, G.; Katovic, A.; Pedulà, V. Performances of Fe-[Al, B]MFI catalysts in benzene hydroxylation with N2O. Catal. Today 2005, 110, 211–220. [Google Scholar] [CrossRef]
- Katovic, A.; Giordano, G.; Bonelli, B.; Onida, B.; Garrone, E.; Lentz, P.; Nagy, J.B. Preparation and characterization of mesoporous molecular sives containing Al, Fe or Zn. Microporous Mesoporous Mater. 2001, 44, 275–281. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [Green Version]
- Migliori, M.; Aloise, A.; Catizzone, E.; Giordano, G. Kinetic Analysis of Methanol to Dimethyl Ether Reaction over H-MFI Catalyst. Ind. Eng. Chem. Res. 2014, 53, 14885–14891. [Google Scholar] [CrossRef]
- Hong, Z.-S.; Cao, Y.; Deng, J.-F.; Fan, K. CO2 Hydrogenation to Methanol Over Cu/ZnO/Al2O3 Catalysts Prepared by a Novel Gel-Network-Coprecipitation Method. Catal. Lett. 2002, 82, 37–44. [Google Scholar] [CrossRef]
- Liang, X.-L.; Dong, X.; Lin, G.-D.; Zhang, H.-B. Carbon nanotube-supported Pd-ZnO catalyst for hydrogenation of CO2 to methanol. Appl. Catal. B Environ. 2009, 88, 315–322. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Liu, X.; Pan, X.; Pei, G.; Huang, Y.; Wang, X.; Zhang, T.; Geng, H. Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst structure dependence of methanol selectivity. Appl. Catal. A Gen. 2016, 514, 51–59. [Google Scholar] [CrossRef]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd–Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2015, 170, 173–185. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Schumann, J.; Tarasov, A.V.; Thomas, N.; Schlögl, R.; Behrens, M. Cu,Zn-based catalysts for methanol synthesis: On the effect of calcination conditions and the part of residual carbonates. Appl. Catal. A Gen. 2016, 516, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Bonura, G.; Arena, F.; Mezzatesta, G.; Cannilla, C.; Spadaro, L.; Frusteri, L. Role of the ceria promoter and carrier on the functionality of Cu-based catalysts in the CO2-to-methanol hydrogenation reaction. Catal. Today 2011, 171, 251–256. [Google Scholar] [CrossRef]
- Wang, G.; Zuo, Y.; Han, M.; Wang, J. Copper crystallite size and methanol synthesis catalytic property of Cu-based catalysts promoted by Al, Zr and Mn. React. Kinet. Mech. Catal. 2010, 101, 443–454. [Google Scholar] [CrossRef]
- Ladera, R.; Pérez-Alonso, F.J.; Carballo, J.M.G.; Ojeda, M.; Rojas, S.; Fierro, J.L.G. Catalytic valorization of CO2 via methanol synthesis with Ga-promoted Cu–ZnO–ZrO2 catalysts. Appl. Catal. B Environ. 2013, 142, 241–248. [Google Scholar] [CrossRef]
- Catizzone, E.; Van Daele, S.; Bianco, M.; Di Michele, A.; Aloise, A.; Migliori, M.; Valtchev, V.; Giordano, G. Catalytic application of ferrierite nanocrystals in vapour-phase dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2019, 243, 273–282. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Aloise, A.; Bonura, G.; Gómez-Hortigüela, L.; Frusteri, L.; Cannilla, C.; Frusteri, F.; Giordano, G. New insights about coke deposition in methanol-to-DME reaction over MOR-, MFI- and FER-type zeolites. J. Ind. Eng. Chem. 2018, 68, 196–208. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Ferrante, G.D.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176, 522–531. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2020, 345, 175–182. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogenation reaction: New evidences of a superior behaviour of FER-based hybrid systems to obtain high DME yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonura, G.; Frusteri, F.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Catalytic features of CuZnZr–zeolite hybrid systems for the direct CO2-to-DME hydrogenation reaction. Catal. Today 2016, 277, 45–54. [Google Scholar] [CrossRef]
- Bonura, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Catizzone, E.; Giordano, G.; Frusteri, F. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J. CO2 Util. 2018, 24, 398–406. [Google Scholar] [CrossRef]
- Miletto, I.; Catizzone, E.; Bonura, G.; Ivaldi, C.; Migliori, M.; Gianotti, E.; Marchese, L.; Frusteri, F.; Giordano, G. In situ FT-IR characterization of CuZnZr/ferrierite hybrid catalysts for one-pot CO2-to-DME conversion. Materials 2018, 11, 2275. [Google Scholar] [CrossRef] [Green Version]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. The effect of FER zeolite acid sites in methanol-to-dimethyl-ether catalytic dehydration. J. Energy Chem. 2017, 26, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Todorova, T.; Vimont, A.; Ruaux, V.; Qin, Z.; Gilson, J.-P.; Valtchev, V. In situ and post-synthesis control of physicochemical properties of FER-type crystals. Microporous Mesoporous Mater. 2014, 200, 334–342. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Microporous Mesoporous Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Sun, R.; Delidovich, I.; Palkovits, R. Dimethoxymethane as a Cleaner Synthetic Fuel: Synthetic Methods, Catalysts, and Reaction Mechanism. ACS Catal. 2019, 9, 1298–1318. [Google Scholar] [CrossRef] [Green Version]
- Tonner, S.P.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Dehydrogenation of methanol to methyl formate over copper catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 384–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, F.; Fan, X.; Tierney, J.; Wender, I. Coproduction of hydrogen and methyl formate by dehydrogenation of methanol. In Proceedings of the 23rd Annual International Pittsburgh Coal Conference, PCC-Coal-Energy, Environment and Sustainable Development, Cape Town, South Africa, 8–12 August 2006. [Google Scholar]
- Zhang, Y.; Drake, I.J.; Briggs, D.N.; Bell, A.T. Synthesis of dimethyl carbonate and dimethoxy methane over Cu-ZSM-5. J. Catal. 2006, 244, 219–229. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. Dimethyl ether synthesis via methanol dehydration: Effect of zeolite structure. Appl. Catal. A Gen. 2015, 502, 215–220. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.; Zhao, C. Direct Synthesis of Hydrogen and Dimethoxylmethane from Methanol on Copper/Silica Catalysts with Optimal Cu+/Cu0 Sites. ChemCatChem 2018, 10, 1140–1147. [Google Scholar] [CrossRef]
- Matter, P.; Ozkan, U.S. Effect of pretreatment conditions on Cu/Zn/Zr-based catalysts for the steam reforming of methanol to H2. J. Catal. 2005, 234, 463–475. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Wang, L.-C.; Liu, Y.-M.; Wu, G.-S.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Cai, M.; Sushkevich, V.; Moldovan, S.; Ersen, O.; Lancelot, C.; Valtchev, V.; Khodakov, A.Y. The role of external acid sites of ZSM-5 in deactivation of hybrid CuZnAl/ZSM-5 catalyst for direct dimethyl ether synthesis from syngas. Appl. Catal. A Gen. 2014, 486, 266–275. [Google Scholar] [CrossRef]
- García-Trenco, A.; Martinez, A. The influence of zeolite surface-aluminum species on the deactivation of CuZnAl/zeolite hybrid catalysts for the direct DME synthesis. Catal. Today 2014, 227, 144–153. [Google Scholar] [CrossRef]







| Sample | Specific Surface Area (B.E.T.) [m2/g] | Micropore Area [m2/g] | External Surface Area [m2/g] | Pore Volume [cm3/g] | Micropore Volume [cm3/g] |
|---|---|---|---|---|---|
| P_FER | 329 | 291 | 48 | 0.226 | 0.136 |
| P_ZSM5 | 360 | 211 | 149 | 0.194 | 0.094 |
| M_ZSM5_W | 218 | 132 | 87 | 0.184 | 0.061 |
| M_ZSM5_A | 229 | 127 | 101 | 0.271 | 0.059 |
| M_FER_W | 185 | 153 | 32 | 0.156 | 0.071 |
| M_FER_A a | 182 | 151 | 31 | 0.335 | 0.038 |
| Sample | Si/Al [mol/mol] | Cu/Zn [mol/mol] | CZZ/Zeolite [w/w] |
|---|---|---|---|
| P_FER | 8.6 | - | - |
| P_ZSM5 | 18.7 | - | - |
| M_FER_W | 10.0 | 1.94 | 0.98 |
| M_FER_A a | 6 | 2.1 | 0.98 |
| M_ZSM5_A | 23 | 1.87 | 0.97 |
| M_ZSM5_W | 25.9 | 2.02 | 0.99 |
| Sample | Weak Sites [μmolNH3/gcat] a | Xi [-] | T b [°C] | Strong Sites [μmolNH3/gcat] a | Xi [-] | T c [°C] | Total Acidity [μmolNH3/gcat] | R2 |
|---|---|---|---|---|---|---|---|---|
| P_FER | 281 | 0.22 | 272 | 976 | 0.78 | 556 | 1257 | 0.997 |
| P_ZSM5 | 172 | 0.25 | 266 | 513 | 0.75 | 455 | 685 | 0.997 |
| M_FER_W | 184 | 0.40 | 308 | 280 | 0.60 | 398 | 464 | 0.998 |
| M_FER_A d | 229 | 0.60 | 250 | 152 | 0.40 | 500 | 381 | 0.994 |
| M_ZSM5_A | 159 | 0.53 | 275 | 140 | 0.47 | 325 | 299 | 0.998 |
| M_ZSM5_W | 188 | 0.6 | 320 | 129 | 0.40 | 363 | 317 | 0.998 |
| Sample | Eapp [J/mol] | R2 [-] |
|---|---|---|
| P_FER | 18 | 0.967 |
| P_ZSM5 | 21 | 0.984 |
| M_FER_W | 14 | 0.965 |
| M_FER_A | 17 | 0.979 |
| M_ZSM5_A | 17 | 0.946 |
| M_ZSM5_W | 33 | 0.993 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliori, M.; Condello, A.; Dalena, F.; Catizzone, E.; Giordano, G. CuZnZr-Zeolite Hybrid Grains for DME Synthesis: New Evidence on the Role of Metal-Acidic Features on the Methanol Conversion Step. Catalysts 2020, 10, 671. https://doi.org/10.3390/catal10060671
Migliori M, Condello A, Dalena F, Catizzone E, Giordano G. CuZnZr-Zeolite Hybrid Grains for DME Synthesis: New Evidence on the Role of Metal-Acidic Features on the Methanol Conversion Step. Catalysts. 2020; 10(6):671. https://doi.org/10.3390/catal10060671
Chicago/Turabian StyleMigliori, Massimo, Antonio Condello, Francesco Dalena, Enrico Catizzone, and Girolamo Giordano. 2020. "CuZnZr-Zeolite Hybrid Grains for DME Synthesis: New Evidence on the Role of Metal-Acidic Features on the Methanol Conversion Step" Catalysts 10, no. 6: 671. https://doi.org/10.3390/catal10060671
APA StyleMigliori, M., Condello, A., Dalena, F., Catizzone, E., & Giordano, G. (2020). CuZnZr-Zeolite Hybrid Grains for DME Synthesis: New Evidence on the Role of Metal-Acidic Features on the Methanol Conversion Step. Catalysts, 10(6), 671. https://doi.org/10.3390/catal10060671