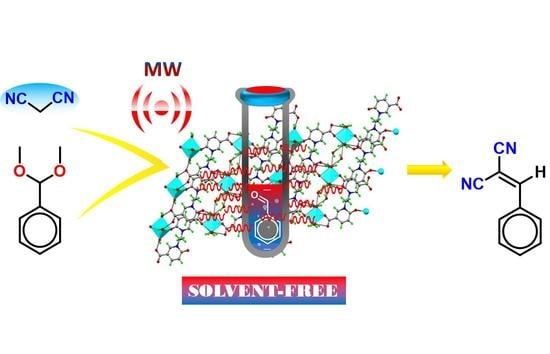
1D Zn(II) Coordination Polymers as Effective Heterogeneous Catalysts in Microwave-Assisted Single-Pot Deacetalization-Knoevenagel Tandem Reactions in Solvent-Free Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization
2.2. Crystal Structure Analysis of 1 and 2
2.3. Topological Analysis of 1 and 2
2.4. Thermogravimetric Analysis of 1 and 2
2.5. Catalytic Activity
3. Materials and Methods
3.1. Synthesis and Characterization
3.1.1. Synthesis of 1,1′-(ethane-1,2-diyl)bis(6-oxo-1,6-dihydropyridine-3-carboxylic acid) (H2L1)
3.1.2. Synthesis of 1,1′-(propane-1,3-diyl)bis(6-oxo-1,6-dihydropyridine-3-carboxylic acid) (H2L2)
3.1.3. Synthesis of [Zn(L1)(H2O)4]n.nH2O (1)
3.1.4. Synthesis of [Zn(L2)(H2O)2]n (2)
3.2. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ghasempour, H.; Wang, K.Y.; Powell, J.A.; ZareKarizi, F.; Lv, X.L.; Morsali, A.; Zhou, H.C. Metal–organic frameworks based on multicarboxylate linkers. Coord. Chem. Rev. 2021, 426, 213542. [Google Scholar] [CrossRef]
- Kumar, N.; Wang, S.Q.; Mukherjee, S.; Bezrukov, A.A.; Patyk-Kaźmierczak, E.; O’Nolan, D.; Kumar, A.; Yu, M.H.; Chang, Z.; Bu, X.H.; et al. Crystal engineering of a rectangular sql coordination network to enable xylenes selectivity over ethylbenzene. Chem. Sci. 2020, 11, 6889–6895. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Gao, E.Q. Azide and carboxylate as simultaneous coupler for magnetic coordination polymers. Coord. Chem. Rev. 2019, 382, 1–31. [Google Scholar] [CrossRef]
- Wang, S.D.; Xie, L.X.; Zhao, Y.F.; Wang, Y.N. A dual luminescent sensor coordination polymer for simultaneous determination of ascorbic acid and tryptophan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118750. [Google Scholar] [CrossRef]
- Paul, A.; Das, K.; Karmakar, A.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. A mechanistic insight into the rapid and selective removal of Congo Red by an amide functionalised Zn(ii) coordination polymer. Dalton Trans. 2020, 49, 12970–12984. [Google Scholar] [CrossRef]
- Tu, Q.Q.; Ren, L.L.; Cui, Y.Y.; Cheng, A.L.; Gao, E.Q. Assembly of four new cobalt coordination polymers modulated by N-coligands: Sensitive and selective sensing of nitroaromatics, Fe3+ and Cr2O72- in water. CrystEngComm 2020, 22, 1789–1801. [Google Scholar] [CrossRef]
- Li, B.; Yan, Q.Q.; Yong, G.P. A new porous coordination polymer reveals selective sensing of Fe3+, Cr2O72−, CrO42−, MnO4− and nitrobenzene, and stimuli-responsive luminescence color conversions. J. Mater. Chem. C 2020, 8, 11786–11795. [Google Scholar] [CrossRef]
- Biradha, K.; Goswami, A.; Moi, R. Coordination polymers as heterogeneous catalysts in hydrogen evolution and oxygen evolution reactions. Chem. Commun. 2020, 56, 10824–10842. [Google Scholar] [CrossRef]
- Wang, J.; Qi, T.; Li, Z.; She, W.; Li, X.; Li, J.; Yan, P.; Li, W.; Li, G. A strategy of two-step tandem catalysis towards direct N-alkylation of nitroarenes with ethanol via facile fabricated novel Co-based catalysts derived from coordination polymers. J. Catal. 2019, 376, 106–118. [Google Scholar] [CrossRef]
- Bhaskaran; Trivedi, M.; Yadav, A.K.; Singh, G.; Kumar, A.; Kumar, G.; Husain, A.; Rath, N.P. Synthetic, spectral, structural and catalytic activity of infinite 3-D and 2-D copper(ii) coordination polymers for substrate size-dependent catalysis for CO2 conversion. Dalton Trans. 2019, 48, 10078–10088. [Google Scholar] [CrossRef]
- Gu, J.; Wen, M.; Cai, Y.; Shi, Z.; Nesterov, D.S.; Kirillova, M.V.; Kirillov, A.M. Cobalt(II) Coordination Polymers Assembled from Unexplored Pyridine-Carboxylic Acids: Structural Diversity and Catalytic Oxidation of Alcohols. Inorg. Chem. 2019, 58, 5875–5885. [Google Scholar] [CrossRef]
- Markad, D.; Khullar, S.; Mandal, S.K. Engineering a Nanoscale Primary Amide-Functionalized 2D Coordination Polymer as an Efficient and Recyclable Heterogeneous Catalyst for the Knoevenagel Condensation Reaction. ACS Appl. Nano Mater. 2018, 1, 5226–5236. [Google Scholar] [CrossRef]
- Karmakar, A.; Pombeiro, A.J.L. Recent advances in amide functionalized metal organic frameworks for heterogeneous catalytic applications. Coord. Chem. Rev. 2019, 395, 86–129. [Google Scholar] [CrossRef]
- Banerjee, D.; Finkelstein, J.; Smirnov, A.; Forster, P.M.; Borkowski, L.A.; Teat, S.J.; Parise, J.B. Synthesis and structural characterization of magnesium based coordination networks in different solvents. Cryst. Growth Des. 2011, 11, 2572–2579. [Google Scholar] [CrossRef]
- Chen, J.; Ohba, M.; Zhao, D.; Kaneko, W.; Kitagawa, S. Polynuclear core-based nickel 1,4-cyclohexanedicarboxylate coordination polymers as temperature-dependent hydrothermal reaction products. Cryst. Growth Des. 2006, 6, 664–668. [Google Scholar] [CrossRef]
- Wang, J.K.; Wang, X.W.; Wang, Z.S.; Yao, L.S.; Niu, L.Z.; Yu, Y.H.; Gao, J.S. Two zinc coordination polymers constructed by 4′-hydroxy-[1,1′-biphenyl]-3,5-dicarboxylic acid (H2BDA) and 4-hydroxy-[1,1′-biphenyl]-3,3′,5,5′-tetracarboxylic acid (H3BTA): Synthesis, structures and luminescence identifying properties. Polyhedron 2019, 167, 85–92. [Google Scholar] [CrossRef]
- Sahu, J.; Ahmad, M.; Bharadwaj, P.K. Structural diversity and luminescence properties of coordination polymers built with a rigid linear dicarboxylate and Zn(II)/Pb(II) ion. Cryst. Growth Des. 2013, 13, 2618–2627. [Google Scholar] [CrossRef]
- Karra, J.R.; Huang, Y.G.; Walton, K.S. Synthesis, characterization, and adsorption studies of nickel(II), zinc(II), and magnesium(II) coordination frameworks of BTTB. Cryst. Growth Des. 2013, 13, 1075–1081. [Google Scholar] [CrossRef]
- Zheng, S.L.; Yang, J.H.; Yu, X.L.; Chen, X.M.; Wong, W.T. Syntheses, Structures, Photoluminescence, and Theoretical Studies of d 10 Metal Complexes of 2,2′-Dihydroxy-[1,1′]binaphthalenyl-3,3′-dicarboxylate. Inorg. Chem. 2004, 43, 830–838. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Yan, T.H.; Zhou, H.C. Seed-mediated evolution of hierarchical metal-organic framework quaternary superstructures. Chem. Sci. 2020, 11, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Chainok, K.; Ponjan, N.; Theppitak, C.; Khemthong, P.; Kielar, F.; Dungkaew, W.; Zhou, Y.; Batten, S.R. Temperature-dependent 3D structures of lanthanide coordination polymers based on dicarboxylate mixed ligands. CrystEngComm 2018, 20, 7446–7457. [Google Scholar] [CrossRef]
- Chen, M.; Hu, M.; Zhao, H.; Tian, J.Y.; Liu, C. Sen Solvent-Controlled Construction of Two 3D Manganese(II) Coordination Polymers Based on Flexible Tripodal Multicarboxylate Linker and Rod-Shaped SBUs. Zeitschrift Anorg. Allg. Chem. 2016, 642, 778–784. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, Y.; Zhao, B.; You, L.; Ren, B.; He, Y.; Wang, S.; Sun, Y. Temperature-tuned topologies and interpenetrations of two 3D porous copper(II)-organic frameworks and gas adsorption behaviors. Inorganica Chim. Acta 2018, 471, 180–185. [Google Scholar] [CrossRef]
- Jarrah, N.; Troyano, J.; Carné-Sánchez, A.; Imaz, I.; Tangestaninejad, S.; Moghadam, M.; Maspoch, D. Dynamic porous coordination polymers built-up from flexible 4,4′-dithiodibenzoate and rigid N-based ligands. Dalton Trans. 2020, 49, 13142–13151. [Google Scholar] [CrossRef]
- Nandi, S.; De Luna, P.; Maity, R.; Chakraborty, D.; Daff, T.; Burns, T.; Woo, T.K.; Vaidhyanathan, R. Imparting gas selective and pressure dependent porosity into a non-porous solid: Via coordination flexibility. Mater. Horiz. 2019, 6, 1883–1891. [Google Scholar] [CrossRef]
- Hawes, C.S.; Chilton, N.F.; Moubaraki, B.; Knowles, G.P.; Chaffee, A.L.; Murray, K.S.; Batten, S.R.; Turner, D.R. Coordination polymers from a highly flexible alkyldiamine-derived ligand: Structure, magnetism and gas adsorption studies. Dalton Trans. 2015, 44, 17494–17507. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X.; Zou, H.; Qi, Y.; Chen, R.; Zhao, L.; Liu, C. A series of coordination polymers assembled from 9,9-dimethylfluorene-2,7- dicarboxylic acid and various flexible bis(imidazole) ligands: Synthesis, structures and properties. CrystEngComm 2014, 16, 7459–7468. [Google Scholar] [CrossRef]
- Colón, Y.J.; Furukawa, S. Understanding the role of linker flexibility in soft porous coordination polymers. Mol. Syst. Des. Eng. 2020, 5, 284–293. [Google Scholar] [CrossRef]
- Kim, H.C.; Huh, S.; Kim, J.Y.; Moon, H.R.; Lee, D.N.; Kim, Y. Zn-MOFs containing flexible α,ω-alkane (or alkene)-dicarboxylates with 1,2-bis(4-pyridyl)ethylene: Comparison with Zn-MOFs containing 1,2-bis(4-pyridyl)ethane ligands. CrystEngComm 2017, 19, 99–109. [Google Scholar] [CrossRef]
- Hwang, I.H.; Kim, H.Y.; Lee, M.M.; Na, Y.J.; Kim, J.H.; Kim, H.C.; Kim, C.; Huh, S.; Kim, Y.; Kim, S.J. Zn-MOFs containing flexible α,ω-alkane (or alkene)- dicarboxylates and 1,2-bis(4-pyridyl)ethane ligands: CO2 sorption and photoluminescence. Cryst. Growth Des. 2013, 13, 4815–4823. [Google Scholar] [CrossRef]
- Ma, L.; Wu, H.; Yang, J.; Liu, Y.Y.; Ma, J.F. Syntheses, crystal structures and knoevenagel condensation reactions of three coordination polymers assembled with Lewis basic ligand. Polyhedron 2018, 144, 6–10. [Google Scholar] [CrossRef]
- Gupta, M.; De, D.; Pal, S.; Pal, T.K.; Tomar, K. A porous two-dimensional Zn(II)-coordination polymer exhibiting SC-SC transmetalation with Cu(II): Efficient heterogeneous catalysis for the Henry reaction and detection of nitro explosives. Dalton Trans. 2017, 46, 7619–7627. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Wang, X.; Gao, K.; Wu, J.; Hou, H.; Fan, Y. Surfactant-Assisted Nanocrystalline Zinc Coordination Polymers: Controlled Particle Sizes and Synergistic Effects in Catalysis. Chem. A Eur. J. 2016, 22, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.P.; Li, Z.H.; Zhang, T.; Cui, J.J.; Gao, Y.; Yao, J.X. Construction of two Zn(ii)/Cd(ii) multifunctional coordination polymers with mixed ligands for catalytic and sensing properties. New J. Chem. 2018, 42, 14203–14209. [Google Scholar] [CrossRef]
- Manuscript, A.; Society, R.; Manuscripts, A.; Manuscript, T.A.; Manuscripts, A.; Society, R.; Manuscript, A. Amide Functionalized Metal Organic Frameworks for Diastereoselective Nitroaldol (Henry) Reaction in Aqueous Medium. RSC Adv. 2015, 5, 87400–87410. [Google Scholar]
- Paul, A.; Martins, L.M.D.R.S.; Karmakar, A.; Kuznetsov, M.L.; Novikov, A.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Environmentally benign benzyl alcohol oxidation and C-C coupling catalysed by amide functionalized 3D Co(II) and Zn(II) metal organic frameworks. J. Catal. 2020, 385, 324–337. [Google Scholar] [CrossRef]
- Karmakar, A.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Zinc metal-organic frameworks: Efficient catalysts for the diastereoselective Henry reaction and transesterification. Dalton Trans. 2014, 43, 7795–7810. [Google Scholar] [CrossRef]
- Karmakar, A.; Guedes Da Silva, M.F.C.; Hazra, S.; Pombeiro, A.J.L. Zinc amidoisophthalate complexes and their catalytic application in the diastereoselective Henry reaction. New J. Chem. 2015, 39, 3004–3014. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Cascade Reactions Catalyzed by Metal Organic Frameworks. ChemSusChem 2014, 7, 2392–2410. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Rúbio, G.M.D.M.; Soliman, M.M.A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Highly Efficient Bifunctional Amide Functionalized Zn and Cd Metal Organic Frameworks for One-Pot Cascade Deacetalization–Knoevenagel Reactions. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmakar, A.; Soliman, M.M.A.; Rúbio, G.M.D.M.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis and catalytic activities of a Zn(ii) based metallomacrocycle and a metal-organic framework towards one-pot deacetalization-Knoevenagel tandem reactions under different strategies: A comparative study. Dalton Trans. 2020, 49, 8075–8085. [Google Scholar] [CrossRef] [PubMed]
- Toyao, T.; Fujiwaki, M.; Horiuchi, Y.; Matsuoka, M. Application of an amino-functionalised metal-organic framework: An approach to a one-pot acid-base reaction. RSC Adv. 2013, 3, 21582–21587. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhou, Y.X.; Wang, H.; Lu, J.; Uchida, T.; Xu, Q.; Yu, S.H.; Jiang, H.L. Multifunctional PdAg@MIL-101 for One-Pot Cascade Reactions: Combination of Host-Guest Cooperation and Bimetallic Synergy in Catalysis. ACS Catal. 2015, 5, 2062–2069. [Google Scholar] [CrossRef]
- Liu, H.; Xi, F.G.; Sun, W.; Yang, N.N.; Gao, E.Q. Amino- and sulfo-bifunctionalized metal-organic frameworks: One-pot tandem catalysis and the catalytic sites. Inorg. Chem. 2016, 55, 5753–5755. [Google Scholar] [CrossRef]
- Mistry, S.; Sarkar, A.; Natarajan, S. New Bifunctional Metal-Organic Frameworks and Their Utilization in One-Pot Tandem Catalytic Reactions. Cryst. Growth Des. 2019, 19, 747–755. [Google Scholar] [CrossRef]
- Yaghi, M.O. Reticular Chemistry Structure Resource; Arizona State University: Tempe, AZ, USA, 2005; Available online: Http://rcsr.anu.edu.au/ (accessed on 18 December 2020).
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr Comput. Comm. Newslett. 2006, 7, 4. Available online: http://www.topos.ssu.samara (accessed on 18 December 2020).
- Blatov, V.A. Nanocluster analysis of intermetallic structures with the program package TOPOS. Struct. Chem. 2012, 23, 955–963. [Google Scholar] [CrossRef]
- Paul, A.; Martins, L.M.D.R.S.; Karmakar, A.; Kuznetsov, M.L.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Zn(II)-to-Cu(II) Transmetalation in an Amide Functionalized Complex and Catalytic Applications in Styrene Oxidation and Nitroaldol Coupling. Molecules 2020, 25, 2644. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Mu, M.; Yan, X.; Li, Y.; Chen, L. Post-modified acid-base bifunctional MIL-101(Cr) for one-pot deacetalization-Knoevenagel reaction. J. Nanoparticle Res. 2017, 19, 148. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Feng, P. Bifunctional heterometallic metal-organic frameworks for solvent-free heterogeneous cascade catalysis. Catalysts 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Sun, F.; Aguila, B.; Perman, J.A.; Ma, S.; Zhu, G. A bifunctional metal—Organic framework featuring the combination of open metal sites and Lewis basic sites for selective gas adsorption and heterogeneous cascade catalysis. J. Mater. Chem. A 2016, 4, 15240–15246. [Google Scholar] [CrossRef]
- RSC Advances. Bruker, APEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]











| Entry | Catalyst | Time (h) | Amount of Catalyst (mol%) | T (°C) | Solvent | Relative Amount of Unreacted A (%) b | Yield of B (%) b | Yield of C (%) b |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 4 | 3 | 80 | Solvent free | 7 | 2 | 91 |
| 2 | 2 | 4 | 3 | 80 | Solvent free | 10 | 3 | 87 |
| 3 | 1 | 0.5 | 3 | 80 | Solvent free | 76 | 15 | 9 |
| 4 | 1 | 1 | 3 | 80 | Solvent free | 62 | 11 | 27 |
| 5 | 1 | 2 | 3 | 80 | Solvent free | 40 | 11 | 49 |
| 6 | 1 | 3 | 3 | 80 | Solvent free | 12 | 9 | 79 |
| 7 | 1 | 4 | 3 | 80 | DMF | 21 | 32 | 47 |
| 8 | 1 | 4 | 3 | 80 | THF | 46 | 22 | 32 |
| 9 | 1 | 4 | 3 | 80 | DMSO | 25 | 30 | 45 |
| 10 | 1 | 4 | 3 | 80 | CH3CN | 38 | 41 | 21 |
| 11 | 1 | 4 | 1 | 80 | Solvent free | 46 | 20 | 34 |
| 12 | 1 | 4 | 2 | 80 | Solvent free | 31 | 13 | 56 |
| 13 | 1 | 4 | 5 | 80 | Solvent free | 16 | 6 | 78 |
| 14 | 1 | 4 | 3 | 25 | Solvent free | 66 | 24 | 10 |
| 15 | 1 | 4 | 3 | 50 | Solvent free | 42 | 12 | 46 |
| 16 | 1 | 4 | 3 | 100 | Solvent free | 8 | 3 | 89 |
| 17 | Blank | 4 | - | 80 | Solvent free | 78 | 22 | 0 |
| 18 | Zn(NO3)2.6H2O | 4 | 3 | 80 | Solvent free | 61 | 32 | 7 |
| 19 | H2L1 | 4 | 3 | 80 | Solvent free | 95 | 5 | 0 |
| 20 | H2L2 | 4 | 3 | 80 | Solvent free | 96 | 4 | 0 |
| Entry | Catalyst | Solvent/Temp/Time | Yield (%) | Ref |
|---|---|---|---|---|
| 1 | 1 | Solvent-free/80 °C/4 h | 91 | This work |
| 2 | 2 | Solvent-free/80 °C/4 h | 87 | This work |
| 3 | [Zn2(L′)(H2O)4]n·4n(H2O) | DMF/75 °C/3 h | 99 | 41 |
| 4 | [Zn4(TBCB)(H2O)6]n.5n(DMAc) | 1,4-dioxane/90 °C/4 h | 99 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, A.; Karmakar, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. 1D Zn(II) Coordination Polymers as Effective Heterogeneous Catalysts in Microwave-Assisted Single-Pot Deacetalization-Knoevenagel Tandem Reactions in Solvent-Free Conditions. Catalysts 2021, 11, 90. https://doi.org/10.3390/catal11010090
Paul A, Karmakar A, Guedes da Silva MFC, Pombeiro AJL. 1D Zn(II) Coordination Polymers as Effective Heterogeneous Catalysts in Microwave-Assisted Single-Pot Deacetalization-Knoevenagel Tandem Reactions in Solvent-Free Conditions. Catalysts. 2021; 11(1):90. https://doi.org/10.3390/catal11010090
Chicago/Turabian StylePaul, Anup, Anirban Karmakar, M. Fátima C. Guedes da Silva, and Armando J. L. Pombeiro. 2021. "1D Zn(II) Coordination Polymers as Effective Heterogeneous Catalysts in Microwave-Assisted Single-Pot Deacetalization-Knoevenagel Tandem Reactions in Solvent-Free Conditions" Catalysts 11, no. 1: 90. https://doi.org/10.3390/catal11010090
APA StylePaul, A., Karmakar, A., Guedes da Silva, M. F. C., & Pombeiro, A. J. L. (2021). 1D Zn(II) Coordination Polymers as Effective Heterogeneous Catalysts in Microwave-Assisted Single-Pot Deacetalization-Knoevenagel Tandem Reactions in Solvent-Free Conditions. Catalysts, 11(1), 90. https://doi.org/10.3390/catal11010090