Synthesis of Thin Titania Coatings onto the Inner Surface of Quartz Tubes and Their Photoactivity in Decomposition of Methylene Blue and Rhodamine B
Abstract
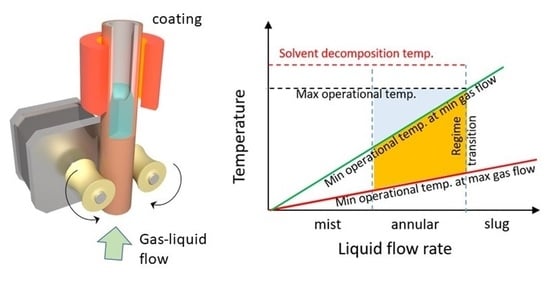
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J. Photocatalytic degradation of rhodamine B catalyzed by TiO2 films on a capillary column. RSC Adv. 2018, 8, 11921–11929. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fu, Y.; Wu, Y.; Zhang, Y.N.; Zuo, T. Low-Cost Y-Doped TiO2 Nanosheets film with highly reactive {001} facets from CRT waste and enhanced photocatalytic removal of Cr(VI) and methyl orange. ACS Sustain. Chem. Eng. 2016, 4, 1794–1803. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, Y.; Zhao, L.; Wang, H.; Song, G.; Tang, G. Microencapsulated phase change materials with TiO2-doped PMMA shell for thermal energy storage and UV-shielding. Sol. Energy Mater. Sol. Cells 2017, 168, 62–68. [Google Scholar] [CrossRef]
- Endres, P.J.; Paunesku, T.; Vogt, S.; Meade, T.J.; Woloschak, G.E. DNA-TiO2 nanoconjugates labeled with magnetic resonance contrast agents. J. Am. Chem. Soc. 2007, 129, 15760–15761. [Google Scholar] [CrossRef] [PubMed]
- Fitra, M.; Daut, I.; Irwanto, M.; Gomesh, N.; Irwan, Y.M. Effect of TiO2 thickness dye solar cell on charge generation. Energy Procedia 2013, 36, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhu, S.; Pan, N. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Cardoso, B.N.; Kohlrausch, E.C.; Laranjo, M.T.; Benvenutti, E.V.; Balzaretti, N.M.; Arenas, L.T.; Santos, M.J.L.; Costa, T.M.H. Tuning anatase-rutile phase transition temperature: TiO2/SiO2 nanoparticles applied in dye-sensitized solar cells. Int. J. Photoenergy 2019, 2019, 7183978. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M. Photodegradation of rhodamine B in water assisted by titania nanorod thin films subjected to various thermal treatments. Environ. Sci. Technol. 2007, 41, 1723–1728. [Google Scholar] [CrossRef]
- Crişan, M.; Mardare, D.; Ianculescu, A.; Drăgan, N.; Niţoi, I.; Crişan, D.; Voicescu, M.; Todan, L.; Oancea, P.; Adomniţei, C.; et al. Iron doped TiO2 films and their photoactivity in nitrobenzene removal from water. Appl. Surf. Sci. 2018, 455, 201–215. [Google Scholar] [CrossRef]
- Bisen, N.; Shrivastava, P.; Hariprasad, N.; Anju, S.G.; Yesodharan, E.P.; Suguna, Y.; Gaya, U.I.; Abdullah, A.H.; Tseng, T.K.; Lin, Y.S.; et al. Sunlight induced removal of Rhodamine B from water through Semiconductor Photocatalysis: Effects of adsorption, reaction conditions and additives. Int. J. Mol. Sci. 2013, 11, 2336–2361. [Google Scholar]
- Van Viet, P.; Sang, T.T.; Hien, N.Q.; Thi, C.M.; Hieu, L. Synthesis of a silver/TiO2 nanotube nanocomposite by gamma irradiation for enhanced photocatalytic activity under sunlight. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 429, 14–18. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Hafizah, N.; Sopyan, I. Cement bonded sol-gel TiO2 powder photocatalysis for phenol removal. Appl. Mech. Mater. 2015, 776, 271–276. [Google Scholar] [CrossRef]
- Allé, P.H.; Fanou, G.D.; Robert, D.; Adouby, K.; Drogui, P. Photocatalytic degradation of Rhodamine B dye with TiO2 immobilized on SiC foam using full factorial design. Appl. Water Sci. 2020, 10, 207. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A.; Stournaras, C.J.; Julbe, A.; Dalmazio, L.; Guizard, C. Evaluation of sol-gel methods for the synthesis of doped-ceria environmental catalysis systems. Part I: Preparation of coatings. J. Eur. Ceram. Soc. 2002, 22, 15–25. [Google Scholar] [CrossRef]
- McCarty, J.G. Kinetics of PdO combustion catalysis. Catal. Today 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Rice, C.V.; Raftery, D. Photocatalytic oxidation of trichloroethylene using TiO2 coated optical microfibers. Chem. Commun. 1999, 10, 895–896. [Google Scholar] [CrossRef]
- Belochapkine, S.; Shaw, J.; Wenn, D.; Ross, J.R.H. The synthesis by deposition-precipitation of porous γ-alumina catalyst supports on glass substrates compatible with microreactor geometries. Catal. Today 2005, 110, 53–57. [Google Scholar] [CrossRef]
- Cini, P.; Blaha, S.R.; Harold, M.P.; Venkataraman, K. Preparation and characterization of modified tubular ceramic membranes for use as catalyst supports. J. Memb. Sci. 1991, 55, 199–225. [Google Scholar] [CrossRef]
- Xiaoding, X.; Vonk, H.; Cybulski, A.; Moulijn, J.A. Alumina washcoating and metal deposition of ceramic monoliths. Stud. Surf. Sci. Catal. 1995, 91, 1069–1078. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Klinger, E.A.; Berenguer-Murcia, A.; Sulman, E.M.; Schouten, J.C. Selective hydrogenation of 2-methyl-3-butyne-2-ol in a wall-coated capillary microreactor with a Pd25Zn75/TiO2 catalyst. Org. Process Res. Dev. 2009, 13, 991–998. [Google Scholar] [CrossRef]
- Rebrov, E.V. Sol-gel synthesis of zeolite coatings and their application in catalytic microstructured reactors. Catal. Ind. 2009, 1, 322–347. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, S.; Wang, X. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]
- Stefanov, P.; Stoychev, D.; Valov, I.; Kakanakova-Georgieva, A.; Marinova, T. Electrochemical deposition of thin zirconia films on stainless steel 316 L. Mater. Chem. Phys. 2000, 65, 222–225. [Google Scholar] [CrossRef]
- Matatov-Meytal, Y.; Barelko, V.; Yuranov, I.; Sheintuch, M. Cloth catalysts in water denitrification. I. Pd on glass fibers. Appl. Catal. B 2000, 27, 127–135. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Matus, E.V.; Yakutova, A.M.; Protasova, L.N.; Ismagilov, I.Z.; Kerzhentsev, M.A.; Rebrov, E.V.; Schouten, J.C. Design of Pt-Sn catalysts on mesoporous titania films for microreactor application. Catal. Today 2009, 147, S81–S86. [Google Scholar] [CrossRef]
- Giornelli, T.; Löfberg, A.; Bordes-Richard, E. Grafting of VOx/TiO2 catalyst on anodized aluminum plates for structured catalytic reactors. Thin Solid Films 2005, 479, 64–72. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Glazneva, T.S.; Berenguer-Murcia, A.; Ismagilov, Z.R.; Schouten, J.C. Control of the thickness of mesoporous titania films for application in multiphase catalytic microreactors. J. Catal. 2010, 271, 161–169. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhao, X.S.; Lee, W.I. Block copolymer-templated synthesis of highly organized mesoporous TiO2-based films and their photoelectrochemical applications. Chem. Eng. J. 2011, 170, 363–380. [Google Scholar] [CrossRef]
- Bravo, J.; Karim, A.; Conant, T.; Lopez, G.P.; Datye, A. Wall coating of a CuO/ZnO/Al2O3 methanol steam reforming catalyst for micro-channel reformers. Chem. Eng. J. 2004, 101, 113–121. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Rebrov, E.V. Novel synthesis of thick wall coatings of titania supported Bi poisoned Pd catalysts and application in selective hydrogenation of acetylene alcohols in capillary microreactors. Lab Chip 2015, 15, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Rebrov, E.V.; Cherkasov, N. Disruptive technology for fine chemicals synthesis with catalyst-coated tube reactors. Chim. Oggi/Chem. Today 2018, 36, 17–20. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Rebrov, E.V. Solvent-free semihydrogenation of acetylene alcohols in a capillary reactor coated with a Pd-Bi/TiO2 catalyst. Appl. Catal. A 2016, 515, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Exposito, A.J.; Bai, Y.; Tchabanenko, K.; Rebrov, E.V.; Cherkasov, N. Process intensification of continuous-flow imine hydrogenation in catalyst-coated tube reactors. Ind. Eng. Chem. Res. 2019, 58, 4433–4442. [Google Scholar] [CrossRef]
- Rebrov, E.V. Two-phase flow regimes in microchannels. Theor. Found. Chem. Eng. 2010, 44, 355–367. [Google Scholar] [CrossRef]
- Warnier, M.J.F. Taylor Flow Hydrodynamics in Gas-Liquid-Solid Micro Reactors; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2009. [Google Scholar]
- Qu, W.; Mudawar, I. Flow boiling heat transfer in two-phase micro-channel heat sinks—II. Annular two-phase flow model. Int. J. Heat Mass Transf. 2003, 46, 2773–2784. [Google Scholar] [CrossRef]
- Yang, F.; Dai, X.; Peles, Y.; Cheng, P.; Khan, J.; Li, C. Flow boiling phenomena in a single annular flow regime in microchannels (I): Characterization of flow boiling heat transfer. Int. J. Heat Mass Transf. 2014, 68, 703–715. [Google Scholar] [CrossRef]
- Abiev, R.S. Hydrodynamics and heat transfer of circulating Two-phase Taylor flow in microchannel heat pipe: Experimental study and Mathematical Model. Ind. Eng. Chem. Res. 2020, 59, 3687–3701. [Google Scholar] [CrossRef]
- Peterson, G.P.; Ma, H.B. Theoretical analysis of the maximum heat transport in triangular grooves: A study of idealized micro heat pipes. J. Heat Transf. 1996, 118, 731–739. [Google Scholar] [CrossRef]
- Helbig, K.; Alexeev, A.; Gambaryan-Roisman, T.; Stephan, P. Evaporation of falling and shear-driven thin films on smooth and grooved surfaces. Flow, Turbul. Combust. 2005, 75, 85–104. [Google Scholar] [CrossRef]
- Sibiryakov, N.; Kabov, O.; Belosludstev, V. Numerical simulation of flow in triangular minichannel. EPJ Web Conf. 2019, 196, 00051. [Google Scholar] [CrossRef] [Green Version]
- Sibiryakov, N.; Kabov, O. Numerical simulation of flow with evaporation in triangular grooves. J. Phys. Conf. Ser. 2019, 1369, 012060. [Google Scholar] [CrossRef]
- Warrier, G.R.; Dhir, V.K.; Momoda, L.A. Heat transfer and pressure drop in narrow rectangular channels. Exp. Therm. Fluid Sci. 2002, 26, 53–64. [Google Scholar] [CrossRef]
- Lazarek, G.M.; Black, S.H. Evaporative heat transfer, pressure drop and critical heat flux in a small vertical tube with R-113. Int. J. Heat Mass Transf. 1982, 25, 945–960. [Google Scholar] [CrossRef]
- Kandlikar, S.G. A general correlation for saturated two-phase flow boiling heat transfer inside horizontal and vertical tubes. J. Heat Transfer 1990, 112, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Winterton, R.H.S. A general correlation for saturated and subcooled flow boiling in tubes and annuli, based on a nucleate pool boiling equation. Int. J. Heat Mass Transf. 1991, 34, 2759–2766. [Google Scholar] [CrossRef]
- Nayar, K.G.; Panchanathan, D.; McKinley, G.H.; Lienhard, J.H. Surface Tension of Seawater. J. Phys. Chem. Ref. Data 2014, 43, 043103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Karapetsas, G.; Valluri, P.; Sefiane, K.; Williams, A.; Takata, Y. Dynamics of hygroscopic aqueous solution droplets undergoing evaporation or vapour absorption. J. Fluid Mech. 2021, 912, 1–30. [Google Scholar] [CrossRef]
- Fukano, T.; Furukawa, T. Prediction of the effects of liquid viscosity on interfacial shear stress and frictional pressure drop in vertical upward gas-liquid annular flow. Int. J. Multiph. Flow 1998, 24, 587–603. [Google Scholar] [CrossRef]
- Magnini, M.; Thome, J.R. A CFD study of the parameters influencing heat transfer in microchannel slug flow boiling. Int. J. Therm. Sci. 2016, 110, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, B.; Banfield, J.F. The size dependence of the surface free energy of titania nanocrystals. Phys. Chem. Chem. Phys. 2009, 11, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Le, H.A.; Linh, L.T.; Chin, S.; Jurng, J. Photocatalytic degradation of methylene blue by a combination of TiO2-anatase and coconut shell activated carbon. Powder Technol. 2012, 225, 167–175. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetlov, S.D.; Sladkovskiy, D.A.; Semikin, K.V.; Utemov, A.V.; Abiev, R.S.; Rebrov, E.V. Synthesis of Thin Titania Coatings onto the Inner Surface of Quartz Tubes and Their Photoactivity in Decomposition of Methylene Blue and Rhodamine B. Catalysts 2021, 11, 1538. https://doi.org/10.3390/catal11121538
Svetlov SD, Sladkovskiy DA, Semikin KV, Utemov AV, Abiev RS, Rebrov EV. Synthesis of Thin Titania Coatings onto the Inner Surface of Quartz Tubes and Their Photoactivity in Decomposition of Methylene Blue and Rhodamine B. Catalysts. 2021; 11(12):1538. https://doi.org/10.3390/catal11121538
Chicago/Turabian StyleSvetlov, Stanislav D., Dmitry A. Sladkovskiy, Kirill V. Semikin, Alexander V. Utemov, Rufat Sh. Abiev, and Evgeny V. Rebrov. 2021. "Synthesis of Thin Titania Coatings onto the Inner Surface of Quartz Tubes and Their Photoactivity in Decomposition of Methylene Blue and Rhodamine B" Catalysts 11, no. 12: 1538. https://doi.org/10.3390/catal11121538
APA StyleSvetlov, S. D., Sladkovskiy, D. A., Semikin, K. V., Utemov, A. V., Abiev, R. S., & Rebrov, E. V. (2021). Synthesis of Thin Titania Coatings onto the Inner Surface of Quartz Tubes and Their Photoactivity in Decomposition of Methylene Blue and Rhodamine B. Catalysts, 11(12), 1538. https://doi.org/10.3390/catal11121538