Assessment on the Effect of Sulfuric Acid Concentration on Physicochemical Properties of Sulfated-Titania Catalyst and Glycerol Acetylation Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of the Synthesized Catalyst
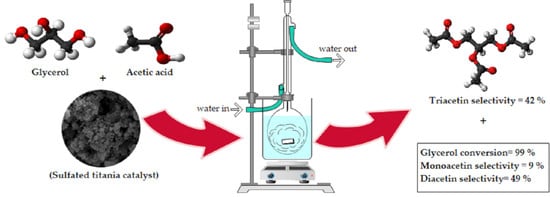
2.2. Catalytic Performance in Glycerol Acetylation
2.3. Catalytic Comparative Study
3. Materials and Method
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Glycerol Acetylation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cannilla, C.; Bonura, G.; Maisano, S.; Frusteri, L.; Migliori, M.; Giordano, G.; Todaro, S.; Frusteri, F. Zeolite-assisted etherification of glycerol with butanol for biodiesel oxygenated additives production. J. Energy Chem. 2020, 48, 136–144. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Subbaramaiah, V.; Hameed, B.H. Lithium loaded coal fly ash as sustainable and effective catalyst for the synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2021, 9, 105999. [Google Scholar] [CrossRef]
- Hu, C.; Yoshida, M.; Chen, H.C.; Tsunekawa, S.; Lin, Y.F.; Huang, J.H. Production of glycerol carbonate from carboxylation of glycerol with CO2 using ZIF-67 as a catalyst. Chem. Eng. Sci. 2021, 235, 116451. [Google Scholar] [CrossRef]
- Azri, N.; Irmawati, R.; Nda-Umar, U.I.; Saiman, M.I.; Taufiq-Yap, Y.H. Promotional effect of transition metals (Cu, Ni, Co, Fe, Zn)–supported on dolomite for hydrogenolysis of glycerol into 1,2-propanediol. Arab. J. Chem. 2021, 14, 103047. [Google Scholar] [CrossRef]
- Raju, N.; Rekha, V.; Abhishek, B.; Kumar, P.M.; Sumana, C.; Lingaiah, N. Studies on continuous selective hydrogenolysis of glycerol over supported Cu-Co bimetallic catalysts. New J. Chem. 2020, 44, 3122–3128. [Google Scholar] [CrossRef]
- Zhan, T.; Liu, W.; Teng, J.; Yue, C.; Li, D.; Wang, S.; Tan, H. Selective oxidation of glycerol to tartronic acid over Pt/N-doped mesoporous carbon with extra framework magnesium catalysts under base-free conditions. Chem. Commun. 2019, 55, 2620–2623. [Google Scholar] [CrossRef]
- Katryniok, B.; Meléndez, R.; Bellière-Baca, V.; Rey, P.; Dumeignil, F.; Fatah, N.; Paul, S. Catalytic dehydration of glycerol to acrolein in a two-zone fluidized bed reactor. Front. Chem. 2019, 7, 127. [Google Scholar] [CrossRef]
- Kansy, D.; Bosowska, K.; Czaja, K.; Poliwoda, A. The formation of glycerol oligomers with two new types of end groups in the presence of a homogeneous alkaline catalyst. Polymers 2019, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Saengarun, C.; Petsom, A.; Tungasmita, D.N. Etherification of glycerol with propylene or 1-butene for fuel additives. Sci. World J. 2017, 2017, 4089036. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.M.; Britto, P.J.; Narula, A.; Saqline, S.; Anand, D.; Bhagyalakshmi, C.; Herle, R.N. Kinetic studies on the synthesis of fuel additives from glycerol using CeO2-ZrO2 metal oxide catalyst. Biofuel Res. J. 2020, 7, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Aghbashlo, M.; Tabatabaei, M.; Rastegari, H.; Ghaziaskar, H.S. Exergy-based sustainability analysis of acetins synthesis through continuous esterification of glycerol in acetic acid using Amberlyst®36 as catalyst. J. Clean. Prod. 2018, 183, 1265–1275. [Google Scholar] [CrossRef]
- Altino, F.M.R.S.; da Silva, D.S.; Bortoluzzi, J.H.; Meneghetti, S.M.P. Investigation of glycerol acetylation in the presence of Sb catalysts. Biomass Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Viswanadham, N.; Saxena, S.K.; Selvamani, A.; Diwakar, J.; Al-Muhtaseb, A.H. Single-pot template-free synthesis of a glycerol-derived C-Si-Zr mesoporous composite catalyst for fuel additive production. New J. Chem. 2020, 44, 8254–8263. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Jothi Ramalingam, R.; Selvaraj, M.; Chia, S.; Tan, S.H.; Khoerunnisa, F.; Ling, T.C.; Ng, E.P. Selective synthesis of triacetyl glyceride biofuel additive via acetylation of glycerol over NiO-supported TiO2 catalyst enhanced by non-microwave instant heating. Appl. Surf. Sci. 2021, 545, 149017. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Maneechakr, P.; Samart, C.; Guan, G. Ultrasound-assisted acetylation of glycerol for triacetin production over green catalyst: A liquid biofuel candidate. Energy Convers. Manag. 2018, 173, 262–270. [Google Scholar] [CrossRef]
- da Silva, D.S.; Altino, F.M.R.S.; Bortoluzzi, J.H.; Meneghetti, S.M.P. Investigation of Sn(IV) catalysts in glycerol acetylation. Mol. Catal. 2020, 494, 111130. [Google Scholar] [CrossRef]
- Sun, J.; Tong, X.; Yu, L.; Wan, J. An efficient and sustainable production of triacetin from the acetylation of glycerol using magnetic solid acid catalysts under mild conditions. Catal. Today 2016, 264, 115–122. [Google Scholar] [CrossRef]
- Silva, L.N.; Gonçalves, V.L.C.; Mota, C.J.A. Catalytic acetylation of glycerol with acetic anhydride. Catal. Commun. 2010, 11, 1036–1039. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Boldrini, D.E. Kinetic study of fuel bio-additive synthesis from glycerol esterification with acetic acid over acid polymeric resin as catalyst. Fuel 2020, 264, 116879. [Google Scholar] [CrossRef]
- Carpegiani, J.A.; Godoy, W.M.; Guimarães, D.H.P.; Aguiar, L.G. Glycerol acetylation catalyzed by an acidic styrene-co-dimethacrylate resin: Experiments and kinetic modeling. React. Kinet. Mech. Catal. 2020, 130, 447–461. [Google Scholar] [CrossRef]
- Mou, R.; Wang, X.; Wang, Z.; Zhang, D.; Yin, Z.; Lv, Y.; Wei, Z. Synthesis of fuel bioadditive by esterification of glycerol with acetic acid over hydrophobic polymer-based solid acid. Fuel 2021, 302, 121175. [Google Scholar] [CrossRef]
- Dizoğlu, G.; Sert, E. Fuel additive synthesis by acetylation of glycerol using activated carbon/UiO-66 composite materials. Fuel 2020, 281, 118584. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Decolatti, H.P.; Querini, C.A.; Dalla Costa, B.O. Hierarchical H-ZSM-5 zeolite and sulfonic SBA-15: The properties of acidic H and behavior in acetylation and alkylation reactions. Microporous. Mesoporous. Mater. 2020, 305, 110284. [Google Scholar] [CrossRef]
- Mertsoy, E.Y.; Sert, E.; Atalay, S.; Atalay, F.S. Fabrication of chromium based metal organic framework (MIL-101)/activated carbon composites for acetylation of glycerol. J. Taiwan Inst. Chem. Eng. 2021, 120, 93–105. [Google Scholar] [CrossRef]
- Spataru, D.; Soares Dias, A.P.; Vieira Ferreira, L.F. Acetylation of biodiesel glycerin using glycerin and glucose derived catalysts. J. Clean. Prod. 2021, 297, 126686. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.B.; Muhamad, E.N.; Azri, N.; Amadi, U.F.; Taufiq-Yap, Y.H. Influence of heterogeneous catalysts and reaction parameters on the acetylation of glycerol to acetin: A review. Appl. Sci. 2020, 10, 7155. [Google Scholar] [CrossRef]
- Cahyono, R.B.; Mufrodi, Z.; Hidayat, A.; Budiman, A. Acetylation of glycerol for triacetin production using Zr-natural zeolite catalyst. ARPN J. Eng. Appl. Sci. 2016, 11, 5194–5197. [Google Scholar]
- Malaika, A.; Kozłowski, M. Glycerol conversion towards valuable fuel blending compounds with the assistance of SO3H-functionalized carbon xerogels and spheres. Fuel Process. Technol. 2019, 184, 19–26. [Google Scholar] [CrossRef]
- Banu, I.; Bumbac, G.; Bombos, D.; Velea, S.; Gălan, A.M.; Bozga, G. Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics. Renew. Energy 2020, 148, 548–557. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Zhao, H.; Hou, Z. Esterification of glycerol with acetic acid over SO3H− functionalized phenolic resin. Fuel 2019, 255, 115842. [Google Scholar] [CrossRef]
- Magar, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C.V. Synthesis and characterization of supported heteropoly acid: Efficient solid acid catalyst for glycerol esterification to produce biofuel additives. Inorg. Nano-Metal Chem. 2020, 50, 1157–1165. [Google Scholar] [CrossRef]
- Beejapur, H.A.; La Parola, V.; Liotta, L.F.; Testa, M.L. Glycerol Acetylation over Organic-Inorganic Sulfonic or Phosphonic Silica Catalysts. ChemistrySelect 2017, 2, 4934–4941. [Google Scholar] [CrossRef]
- Dalla Costa, B.O.; Decolatti, H.P.; Legnoverde, M.S.; Querini, C.A. Influence of acidic properties of different solid acid catalysts for glycerol acetylation. Catal. Today 2017, 289, 222–230. [Google Scholar] [CrossRef]
- V Bokade, V. Synthesis of Oxygenated Fuel Additives via Acetylation of Bio-Glycerol over H2SO4 Modified Montmorillonite K10 Catalyst. Prog. Petrochem. Sci. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R. Archna Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Muhamad, E.N.; Taufiq-Yap, Y.H.; Azri, N. Synthesis and characterization of sulfonated carbon catalysts derived from biomass waste and its evaluation in glycerol acetylation. Biomass Convers. Biorefinery 2020, 40, 437–446. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Almas, Q.; Sievers, C.; Jones, C.W. Role of themesopore generation method in structure, activity and stability of MFI catalysts in glycerol acetylation. Appl. Catal. A Gen. 2019, 571, 107–117. [Google Scholar] [CrossRef]
- Jothi Ramalingam, R.; Radhika, T.; Adam, F.; Dolla, T.H. Acetylation of glycerol over bimetallic Ag–Cu doped rice husk silica based biomass catalyst for bio-fuel additives application. Int. J. Ind. Chem. 2016, 7, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Chipurici, P.; Vlaicu, A.; Calinescu, I.; Vinatoru, M.; Vasilescu, M.; Ignat, N.D.; Mason, T.J. Ultrasonic, hydrodynamic and microwave biodiesel synthesis – A comparative study for continuous process. Ultrason. Sonochem. 2019, 57, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.A.; Pudi, S.M.; Biswas, P. Esterification of glycerol with acetic acid over highly active and stable alumina-based catalysts: A reaction kinetics study. Chem. Biochem. Eng. Q. 2016, 30, 33–45. [Google Scholar] [CrossRef]
- Tomer, R.; Biswas, P. Dehydration of glucose/fructose to 5-hydroxymethylfurfural (5-HMF) over an easily recyclable sulfated titania (SO42-/TiO2) catalyst. New J. Chem. 2020, 44, 20734–20750. [Google Scholar] [CrossRef]
- Geetha, S.; Thangamani, A.; Valliappan, R.; Vedanayaki, S.; Ganapathi, A. Sulfated titania (TiO2-SO42−) as an efficient and reusable solid acid catalyst for the multi-component synthesis of highly functionalized piperidines. Chem. Data Collect. 2020, 30, 100565. [Google Scholar] [CrossRef]
- Patil, S.M.; Deshmukh, S.P.; More, K.V.; Shevale, V.B.; Mullani, S.B.; Dhodamani, A.G.; Delekar, S.D. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2019, 225, 247–255. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles - Synthesized via sol-gel route. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Nachit, W.; Touhtouh, S.; Ramzi, Z.; Zbair, M.; Eddiai, A.; Rguiti, M.; Bouchikhi, A.; Hajjaji, A.; Benkhouja, K. Synthesis of nanosized TiO2 powder by sol gel method at low temperature. Mol. Cryst. Liq. Cryst. 2016, 627, 170–175. [Google Scholar] [CrossRef]
- Humelnicu, A.C.; Samoila, P.; Asandulesa, M.; Cojocaru, C.; Bele, A.; Marinoiu, A.T.; Sacca, A.; Harabagiu, V. Chitosan-sulfated titania composite membranes with potential applications in fuel cell: Influence of cross-linker nature. Polymers 2020, 12, 1125. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Alhassan, S.M.; Mishra, D.K.; Jegal, J.; Hwang, J.S. Solvent free cyclodehydration of sorbitol to isosorbide over mesoporous sulfated titania with enhanced catalytic performance. Mol. Catal. 2018, 454, 77–86. [Google Scholar] [CrossRef]
- Yang, W.; Ok, Y.S.; Dou, X.; Zhang, Y.; Yang, M.; Wei, D.; Xu, P. Effectively remediating spiramycin from production wastewater through hydrolyzing its functional groups using solid superacid TiO2/SO4. Environ. Res. 2019, 175, 393–401. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; Batistella, M.A.; Ulson de Souza, A.A.; Ulson de Souza, S.M.d.A.G. Synthesis of superacid sulfated TiO2 prepared by sol-gel method and its use as a titania precursor in obtaining a kaolinite/TiO2 nano-hybrid composite. Powder Technol. 2021, 381, 366–380. [Google Scholar] [CrossRef]
- Deris, N.H.; Rashid, U.; Soltani, S.; Choong, T.S.Y.; Nehdi, I.A. Study the effect of various sulfonation methods on catalytic activity of carbohydrate-derived catalysts for ester production. Catalysts 2020, 10, 638. [Google Scholar] [CrossRef]
- Liu, G.; Tsen, W.C.; Hu, F.; Zhong, F.; Wang, J.; Liu, H.; Wen, S.; Zheng, G.; Qin, C.; Gong, C. Enhanced proton conductivities of chitosan-based membranes by inorganic solid superacid SO42−–TiO2 coated carbon nanotubes. Int. J. Hydrogen Energy 2020, 45, 29212–29221. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Synthesis of bio-additives: Acetylation of glycerol over zirconia-based solid acid catalysts. Catal. Commun. 2010, 11, 1224–1228. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Irmawati, R.; Muhamad, E.N.; Azri, N.; Ishak, N.S.; Yahaya, M.; Taufiq-Yap, Y.H. Organosulfonic acid-functionalized biomass-derived carbon as a catalyst for glycerol acetylation and optimization studies via response surface methodology. J. Taiwan Inst. Chem. Eng. 2021, 118, 355–370. [Google Scholar] [CrossRef]









| Catalyst | BET Surface Area (m2 g−1) | Pore Volume (nm) | Pore Diameter (nm) |
|---|---|---|---|
| TC | 11.8 | 0.0029 | 5.4 |
| 5SA | 7.4 | 0.0016 | 5.4 |
| 10SA | 6.2 | 0.0014 | 5.2 |
| 15SA | 1.9 | 0.0003 | 5.2 |
| 20SA | 0.2 | 0.00003 | 5.2 |
| Catalyst | XRF Analysis | NH3-TPD Analysis | |
|---|---|---|---|
| TiO2 (%) | SO3 (%) | Amount of NH3 Adsorbed (µmol g−1) * | |
| TC | 99 | - | 12.9 |
| 5SA | 99 | 1 | 28.1 |
| 10SA | 95 | 5 | 279.0 |
| 15SA | 95 | 5 | 342.6 |
| 20SA | 90 | 10 | 444.6 |
| Catalyst | Method | AA | Reaction Parameters | GL (%) | Selectivity (%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | MR | CL (g) | t (h) | MA | DA | TA | |||||
| Blank | Reflux | AAC | 100 | 1:6 | - | 2 | 58 | 51 | 22 | 1 | This work |
| TC (Non sulfated titania) | Reflux | AAC | 100 | 1:6 | 0.5 | 2 | 100 | 22 | 63 | 16 | This work |
| 5SA (sulfated titania) | Reflux | AAC | 100 | 1:6 | 0.5 | 2 | 100 | 7 | 55 | 38 | This work |
| 10SA (sulfated titania) | Reflux | AAC | 100 | 1:6 | 0.5 | 2 | 100 | 8 | 51 | 41 | This work |
| 15SA (sulfated titania) | Reflux | AAC | 100 | 1:6 | 0.5 | 2 | 99 | 9 | 49 | 42 | This work |
| 20SA (sulfated titania) | Reflux | AAC | 100 | 1:6 | 0.5 | 2 | 76 | 10 | 47 | 36 | This work |
| 30NiO/TiO2 | Non- Microwave instant | AAC | 170 | 1:10 | 0.04 | 0.5 | 90 | 20 | 15 | 66 | [14] |
| Fe-Sn-Ti (SO42−)-400 | Autoclave | AAH | 80 | Gly-1.5 g, AAH-8.4g | 0.05 | 0.5 | 100 | 0 | 1 | 99 | [17] |
| MoOx/TiO2-ZrO2 | Reflux | AAC | 120 | 1:6 | 5 wt% | 3 | 100 | 52 | 41 | 8 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shera Farisya, M.R.; Irmawati, R.; Shafizah, I.N.; Taufiq-Yap, Y.H.; Muhamad, E.N.; Lee, S.L.; Salamun, N. Assessment on the Effect of Sulfuric Acid Concentration on Physicochemical Properties of Sulfated-Titania Catalyst and Glycerol Acetylation Performance. Catalysts 2021, 11, 1542. https://doi.org/10.3390/catal11121542
Shera Farisya MR, Irmawati R, Shafizah IN, Taufiq-Yap YH, Muhamad EN, Lee SL, Salamun N. Assessment on the Effect of Sulfuric Acid Concentration on Physicochemical Properties of Sulfated-Titania Catalyst and Glycerol Acetylation Performance. Catalysts. 2021; 11(12):1542. https://doi.org/10.3390/catal11121542
Chicago/Turabian StyleShera Farisya, Mohamad Rasid, Ramli Irmawati, Ishak Nor Shafizah, Yun Hin Taufiq-Yap, Ernee Noryana Muhamad, Siew Ling Lee, and Nurrulhidayah Salamun. 2021. "Assessment on the Effect of Sulfuric Acid Concentration on Physicochemical Properties of Sulfated-Titania Catalyst and Glycerol Acetylation Performance" Catalysts 11, no. 12: 1542. https://doi.org/10.3390/catal11121542
APA StyleShera Farisya, M. R., Irmawati, R., Shafizah, I. N., Taufiq-Yap, Y. H., Muhamad, E. N., Lee, S. L., & Salamun, N. (2021). Assessment on the Effect of Sulfuric Acid Concentration on Physicochemical Properties of Sulfated-Titania Catalyst and Glycerol Acetylation Performance. Catalysts, 11(12), 1542. https://doi.org/10.3390/catal11121542