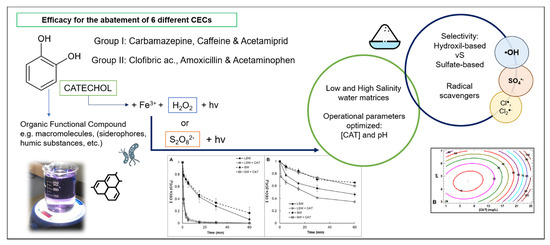
Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Catechol in Photo-Fenton and Persulfate-Based Systems
2.2. Some Insights by Assessing Individual Behavior of the Different CECs
2.3. Effect of Operational Factors and Reagents Consumption
2.3.1. Photo-Fenton System
2.3.2. Photo-Induced Iron Activation of Persulfate
3. Materials and Methods
3.1. Reagents
3.2. Target Solution and Water Matrices
3.3. Experimental Set-up
3.4. Analytical Measurements
3.5. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part II.—The ferric ion reaction. Trans. Faraday Soc. 1951, 47, 591–616. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Pérez-Almeida, N.; González, A.G.; Santana-Casiano, J.M.; González-Dávila, M. Iron and copper redox interactions in UV-seawater: A kinetic model approach. Chem. Geol. 2019, 506, 149–161. [Google Scholar] [CrossRef]
- Lueder, U.; Jørgensen, B.B.; Kappler, A.; Schmidt, C. Photochemistry of iron in aquatic environments. Environ. Sci. Process. Impacts 2020, 22, 12–24. [Google Scholar] [CrossRef]
- Giannakis, S.; López, M.I.P.; Spuhler, D.; Pérez, J.A.S.; Ibáñez, P.F.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction-Part 2: A review of the applications for drinking water and wastewater disinfection. Appl. Catal. B Environ. 2016, 198, 431–446. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Hu, J.; Sans, C. Accelerated degradation of iopamidol in iron activated persulfate systems: Roles of complexing agents. Chem. Eng. J. 2017, 316, 288–295. [Google Scholar] [CrossRef]
- Sandy, M.; Butler, A. Microbial iron acquisition: Marine and terrestrial siderophores. Chem. Rev. 2009, 109, 4580–4595. [Google Scholar] [CrossRef] [Green Version]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbiol. 2012, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avdeef, A.; Sofen, S.R.; Bregante, T.L.; Raymond, K.N. Coordination chemistry of microbial iron transport compounds. 9.1 Stability constants for catechol models of enterobactin. J. Am. Chem. Soc. 1978, 100, 5362–5370. [Google Scholar] [CrossRef]
- Årstøl, E.; Hohmann-Marriott, M.F. Cyanobacterial siderophores—Physiology, structure, biosynthesis, and applications. Mar. Drugs 2019, 17, 281. [Google Scholar] [CrossRef] [Green Version]
- García-Negueroles, P.; García-Ballesteros, S.; Amat, A.M.; Laurenti, E.; Arques, A.; Santos-Juanes, L. Unveiling the dependence between hydroxyl radical generation and performance of fenton systems with complexed iron. ACS Omega 2019, 4, 21698–21703. [Google Scholar] [CrossRef] [PubMed]
- García-Ballesteros, S.; Grimalt, J.; Berto, S.; Minella, M.; Laurenti, E.; Vicente, R.; López-Pérez, M.F.; Amat, A.M.; Bianco Prevot, A.; Arques, A. New route for valorization of oil mill wastes: Isolation of humic-like substances to be employed in solar-driven processes for pollutants removal. ACS Omega 2018, 3, 13073–13080. [Google Scholar] [CrossRef] [PubMed]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.; Teixeira, A.C.S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Whitby, H.; Planquette, H.; Cassar, N.; Bucciarelli, E.; Osburn, C.L.; Janssen, D.J.; Cullen, J.T.; González, A.G.; Völker, C.; Sarthou, G. A call for refining the role of humic-like substances in the oceanic iron cycle. Sci. Rep. 2020, 10, 6144. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, Y.; Xu, J.; Wu, F.; Mailhot, G. Iron(III)-induced photooxidation of arsenite in the presence of carboxylic acids and phenols as model compounds of natural organic matter. Chemosphere 2021, 263, 128142. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, J.; Yu, Q.; Qiu, X.; Sasaki, K. Understanding how specific functional groups in humic acid affect the sorption mechanisms of different calcinated layered double hydroxides. Chem. Eng. J. 2020, 392, 123633. [Google Scholar] [CrossRef]
- Perron, N.R.; Wang, H.C.; Deguire, S.N.; Jenkins, M.; Lawson, M.; Brumaghim, J.L. Kinetics of iron oxidation upon polyphenol binding. Dalt. Trans. 2010, 39, 9982–9987. [Google Scholar] [CrossRef]
- Barbeau, K.; Rue, E.L.; Trick, C.G.; Bruland, K.W.; Butler, A. Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnol. Oceanogr. 2003, 48, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ma, W.; He, J.; Zhao, J. Fenton degradation of malachite green catalyzed by aromatic additives. J. Phys. Chem. A 2002, 106, 9485–9490. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, C.; Liu, H. Fenton-like degradation of dimethyl phthalate enhanced by quinone species. J. Hazard. Mater. 2020, 382, 121007. [Google Scholar] [CrossRef]
- Santana-Casiano, J.M.; González-Dávila, M.; González, A.G.; Millero, F.J. Fe(III) reduction in the presence of Catechol in seawater. Aquat. Geochem. 2010, 16, 467–482. [Google Scholar] [CrossRef]
- Zanta, C.L.P.S.; Friedrich, L.C.; Machulek, A.; Higa, K.M.; Quina, F.H. Surfactant degradation by a catechol-driven Fenton reaction. J. Hazard. Mater. 2010, 178, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Nichela, D.A.; Donadelli, J.A.; Caram, B.F.; Haddou, M.; Rodriguez Nieto, F.J.; Oliveros, E.; García Einschlag, F.S. Iron cycling during the autocatalytic decomposition of benzoic acid derivatives by Fenton-like and photo-Fenton techniques. Appl. Catal. B Environ. 2015, 170–171, 312–321. [Google Scholar] [CrossRef]
- Calza, P.; Campra, L.; Pelizzetti, E.; Minero, C. Role of H2O2 in the photo-transformation of phenol in artificial and natural seawater. Sci. Total Environ. 2012, 431, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofrano, G.; Rizzo, L.; Grassi, M.; Belgiorno, V. Advanced oxidation of catechol: A comparison among photocatalysis, Fenton and photo-Fenton processes. Desalination 2009, 249, 878–883. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, C.; Lyu, S.; Liu, H.; Jiang, C.; Lei, Y. Enhancement of Fenton degradation by catechol in a wide initial pH range. Sep. Purif. Technol. 2016, 169, 202–209. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2019, 2000, 1–24. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- García-Vaquero, N.; Lee, E.; Jiménez Castañeda, R.; Cho, J.; López-Ramírez, J.A. Comparison of drinking water pollutant removal using a nanofiltration pilot plant powered by renewable energy and a conventional treatment facility. Desalination 2014, 347, 94–102. [Google Scholar] [CrossRef]
- Vicente-Cera, I.; Moreno-Andrés, J.; Amaya-Vías, D.; Biel-Maeso, M.; Pintado-Herrera, M.G.; Lara-Martín, P.A.; Acevedo-Merino, A.; López-Ramírez, J.A.; Nebot, E. Chemical and microbiological characterization of cruise vessel wastewater discharges under repair conditions. Ecotoxicol. Environ. Saf. 2019, 169, 68–75. [Google Scholar] [CrossRef]
- Carra, I.; Sánchez Pérez, J.A.; Malato, S.; Autin, O.; Jefferson, B.; Jarvis, P. Performance of different advanced oxidation processes for tertiary wastewater treatment to remove the pesticide acetamiprid. J. Chem. Technol. Biotechnol. 2016, 91, 72–81. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Llorca, M.; Blasco, J.; Barceló, D. Chapter 1—Contaminants in the Marine Environment; Blasco, J., Chapman, P.M., Campana, O., Hampel, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–34. ISBN 9780128033715. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; Chiaia-Hernández, A.C.; Biel-Maeso, M.; Baena-Nogueras, R.M.; Hollender, J. Tracing urban wastewater contaminants into the atlantic ocean by nontarget screening. Environ. Sci. Technol. 2020, 54, 3996–4005. [Google Scholar] [CrossRef]
- Gomis, J.; Bianco Prevot, A.; Montoneri, E.; González, M.C.; Amat, A.M.; Mártire, D.O.; Arques, A.; Carlos, L. Waste sourced bio-based substances for solar-driven wastewater remediation: Photodegradation of emerging pollutants. Chem. Eng. J. 2014, 235, 236–243. [Google Scholar] [CrossRef]
- Wu, Y.; Monfort, O.; Dong, W.; Brigante, M.; Mailhot, G. Enhancement of iron-mediated activation of persulfate using catechin: From generation of reactive species to atenolol degradation in water. Sci. Total Environ. 2019, 697. [Google Scholar] [CrossRef]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Rommozzi, E.; Giannakis, S.; Giovannetti, R.; Vione, D.; Pulgarin, C. Detrimental vs. beneficial influence of ions during solar (SODIS) and photo-Fenton disinfection of E. coli in water: (bi)carbonate, chloride, nitrate and nitrite effects. Appl. Catal. B Environ. 2020, 270, 118877. [Google Scholar] [CrossRef]
- Machulek Amilcar, J.; Moraes Jose, E.F.; Vautier-Giongo, C.; Silverio Cristina, A.; Friedrich Leidi, C.; Nascimento Claudio, A.O.; Gonzalez Monica, C.; Quina Frank, H. Abatement of the inhibitory effect of chloride anions on the photo-Fenton process. Environ. Sci. Technol. 2007, 41, 8459–8463. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Farinango, G.; Romero-Martínez, L.; Acevedo-Merino, A.; Nebot, E. Application of persulfate salts for enhancing UV disinfection in marine waters. Water Res. 2019, 163, 114866. [Google Scholar] [CrossRef] [PubMed]
- Nihemaiti, M.; Miklos, D.B.; Hübner, U.; Linden, K.G.; Drewes, J.E.; Croué, J.P. Removal of trace organic chemicals in wastewater effluent by UV/H2O2and UV/PDS. Water Res. 2018, 145, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Cheng, S.; Luo, N.; Yang, X.; An, T. Rate constants and mechanisms of the reactions of Cl• and Cl2•- with Trace Organic Contaminants. Environ. Sci. Technol. 2019, 53, 11170–11182. [Google Scholar] [CrossRef]
- Ye, T.; Wei, Z.; Spinney, R.; Tang, C.J.; Luo, S.; Xiao, R.; Dionysiou, D.D. Chemical structure-based predictive model for the oxidation of trace organic contaminants by sulfate radical. Water Res. 2017, 116, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Deng, H.; Chu, W.; An, N.; Peng, F. Investigation of clofibric acid removal by UV/persulfate and UV/chlorine processes: Kinetics and formation of disinfection byproducts during subsequent chlor(am)ination. Chem. Eng. J. 2018, 331, 364–371. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Deng, J.; Li, Q.; Chen, W.; Li, G.; Chen, G.; Wang, J. Comparison of acetaminophen degradation in UV-LED-based advance oxidation processes: Reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment. Sci. Total Environ. 2020, 723, 137993. [Google Scholar] [CrossRef]
- Deemter, D.; Oller, I.; Amat, A.M.; Malato, S. Effect of salinity on preconcentration of contaminants of emerging concern by nanofiltration: Application of solar photo-Fenton as a tertiary treatment. Sci. Total Environ. 2020, 756, 143593. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate Constants and Mechanism of Reaction of sulfate radical anion with Aromatic Compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, B.; Bu, Y.; Chen, Y.; Ma, J.; Rosario-Ortiz, F.L.; Zhu, R. Some issues limiting photo(cata)lysis application in water pollutant control: A critical review from chemistry perspectives. Water Res. 2020, 174, 115605. [Google Scholar] [CrossRef] [PubMed]
- Nichela, D.; Haddou, M.; Benoit-Marquié, F.; Maurette, M.T.; Oliveros, E.; García Einschlag, F.S. Degradation kinetics of hydroxy and hydroxynitro derivatives of benzoic acid by fenton-like and photo-fenton techniques: A comparative study. Appl. Catal. B Environ. 2010, 98, 171–179. [Google Scholar] [CrossRef]
- Sciscenko, I.; Garcia-Ballesteros, S.; Sabater, C.; Castillo, M.A.; Escudero-Oñate, C.; Oller, I.; Arques, A. Monitoring photolysis and (solar photo)-Fenton of enrofloxacin by a methodology involving EEM-PARAFAC and bioassays: Role of pH and water matrix. Sci. Total Environ. 2020, 719, 137331. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; García Einschlag, F.S.; Amat, A.M.; Arques, A. Combining ZVI reduction with photo-Fenton process for the removal of persistent pollutants. Chem. Eng. J. 2017, 310, 484–490. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Dos Santos, W.N.L.; Quintella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]





| Humic Like Substances | Concentration of HLS Tested | Operational Variables pH/H2O2 | Target Compounds | Improvement | References |
|---|---|---|---|---|---|
| OMW-HLS | 10 mg L−1 30 mg L−1 60 mg L−1 | pH 5 [H2O2] = 60 mg L−1 | Caffeine | Removal of 95% of initial amount at 10 min with 10 mg L−1 of HLS vs. 45% removal without these substances. | García-Ballesteros et al., 2018 [18] |
| SBO from urban wastes | 30 mg L−1 | pH 5 [H2O2] = 60 mg L−1 | Caffeine | Removal of 100% of initial amount at 90 min. vs. 60% removal without these substances. | García-Negueroles et al., 2019 [17] |
| 10 mg L−1 | pH 5.2 [H2O2] = 75 mg L−1 | Acetaminophen, amoxicillin, caffeine, acetamiprid, carbamazepine and clofibric acid. | Removal of 100% of initial amount of 4 pollutants at t30w = 30 min vs. 80 min (t30w) needed in absence of these substances. | Gomis et al., 2014 [41] |
| Number of Experiments | Coded Values | Operational Variables | t50% (Ʃ CECs), (min) | ||||
|---|---|---|---|---|---|---|---|
| X1 (5 Levels) | X2 (3 Levels) | [CAT] (mg·L−1) | pH | Fe(III)/H2O2 | Fe(III)/S2O82− | ||
| LSW | SW | LSW | |||||
| 1 | 0 | 0 | 13 | 5 | 0.67 | 0.82 | 18.99 |
| 2 | 1.0 | 0 | 25 | 5 | 2.48 | 0.85 | 83.31 |
| 3 | 0.5 | 0.817 | 19 | 7 | 40.31 | 40.49 | 90.16 |
| 4 | −1.0 | 0.000 | 1 | 5 | 9.66 | 22.22 | 34.95 |
| 5 | −0.5 | −0.817 | 7 | 3 | 0.41 | 8.01 | 18.90 |
| 6 | 0.5 | −0.817 | 19 | 3 | 0.39 | 4.16 | 88.96 |
| 7 | −0.5 | 0.817 | 7 | 7 | 82.39 | 76.61 | 43.69 |
| 8 | 0.0 | 0.000 | 13 | 5 | 0.80 | 0.87 | 19.86 |
| 9 | 0.0 | 0.000 | 13 | 5 | 0.76 | 0.82 | 19.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Andrés, J.; Vallés, I.; García-Negueroles, P.; Santos-Juanes, L.; Arques, A. Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties. Catalysts 2021, 11, 372. https://doi.org/10.3390/catal11030372
Moreno-Andrés J, Vallés I, García-Negueroles P, Santos-Juanes L, Arques A. Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties. Catalysts. 2021; 11(3):372. https://doi.org/10.3390/catal11030372
Chicago/Turabian StyleMoreno-Andrés, Javier, Iván Vallés, Paula García-Negueroles, Lucas Santos-Juanes, and Antonio Arques. 2021. "Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties" Catalysts 11, no. 3: 372. https://doi.org/10.3390/catal11030372
APA StyleMoreno-Andrés, J., Vallés, I., García-Negueroles, P., Santos-Juanes, L., & Arques, A. (2021). Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties. Catalysts, 11(3), 372. https://doi.org/10.3390/catal11030372