Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review
Abstract
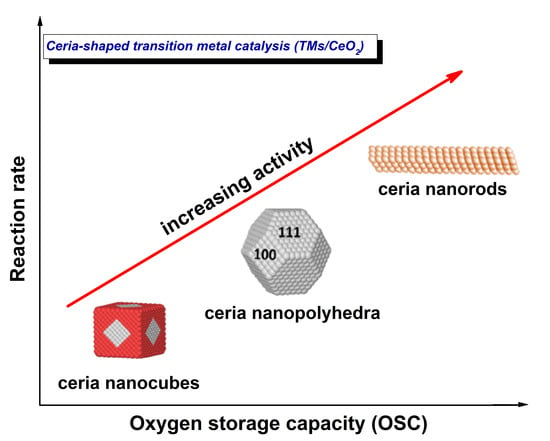
:1. Introduction
- Electronic perturbations linked to bonding interactions between TMs and ceria nanoparticles
- Facilitation of oxygen vacancies’ formation resulting in enhanced reducibility and oxygen exchange kinetics
- High intrinsic activity of interfacial sites
2. Synthesis and Characterization of CeO2 NPs of Different Morphology
3. Ceria Shape Effects on the Structural Defects and Redox Properties
4. Physicochemical Properties of Ceria-Based Transition Metal (Fe, Co, Ni, and Cu) Catalysts
5. Implication in Catalysis
5.1. CO Oxidation
5.2. N2O Decomposition
5.3. CO2 Hydrogenation
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Tang, C.; Gao, F.; Dong, L. Research progress on the catalytic elimination of atmospheric molecular contaminants over supported metal-oxide catalysts. Catal. Sci. Technol. 2014, 4, 2814–2829. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. The role of ceria-based nanostructured materials in energy applications. Mater. Today 2014, 17, 349–357. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y. Copper-based non-precious metal heterogeneous catalysts for environmental remediation. Chin. J. Catal. 2018, 39, 566–582. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Graciani, J.; Senanayake, S.D.; Grinter, D.C.; Stacchiola, D.; Hrbek, J.; Fernández-Sanz, J. Inverse Oxide/Metal Catalysts in Fundamental Studies and Practical Applications: A Perspective of Recent Developments. J. Phys. Chem. Lett. 2016, 7, 2627–2639. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Song, S.; Zhang, H. Syntheses and Applications of Noble-Metal-free CeO2-Based Mixed-Oxide Nanocatalysts. Chem 2019, 5, 1743–1774. [Google Scholar] [CrossRef]
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: Preparation, characterization, and application in energy and environmental catalysis. MRS Commun. 2016, 6, 311–329. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen vacancies in metal oxides: Recent progress towards advanced catalyst design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Shen, W. Shape Engineering of Oxide Nanoparticles for Heterogeneous Catalysis. Chem. Asian J. 2016, 11, 1470–1488. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Recent advances on the rational design of non-precious metal oxide catalysts exemplified by CuOx/CeO2 binary system: Implications of size, shape and electronic effects on intrinsic reactivity and metal-support interactions. Catalysts 2020, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Hermes, E.D.; Jenness, G.R.; Schmidt, J.R. Decoupling the electronic, geometric and interfacial contributions to support effects in heterogeneous catalysis. Mol. Simul. 2015, 41, 123–133. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lu, J. A Review on Particle Size Effect in Metal-Catalyzed Heterogeneous Reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Rudel, H.E.; Lane, M.K.M.; Muhich, C.L.; Zimmerman, J.B. Toward Informed Design of Nanomaterials: A Mechanistic Analysis of Structure-Property-Function Relationships for Faceted Nanoscale Metal Oxides. ACS Nano 2020, 14, 16472–16501. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic Nanoparticles in Heterogeneous Catalysis. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Yang, F.; Deng, D.; Pan, X.; Fu, Q.; Bao, X. Understanding nano effects in catalysis. Natl. Sci. Rev. 2015, 2, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Sun, L.-D.; Yan, C.-H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Grinter, D.C.; Liu, Z.; Palomino, R.M.; Senanayake, S.D. Ceria-based model catalysts: Fundamental studies on the importance of the metal-ceria interface in CO oxidation, the water-gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 2017, 46, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef] [Green Version]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Qin, R.; Zheng, N.; Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 2021, 16, 129–139. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A.C. Surface and redox properties of cobalt-ceria binary oxides: On the effect of Co content and pretreatment conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Kraia, T.; Kaklidis, N.; Konsolakis, M.; Marnellos, G.E. Hydrogen production by H2S decomposition over ceria supported transition metal (Co, Ni, Fe and Cu) catalysts. Int. J. Hydrogen Energy 2019, 44, 9753–9762. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Chen, X.; Konsolakis, M.; Psarras, A.C.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of toluene on Ce-Co and La-Co mixed oxides synthesized by exotemplating and evaporation methods. Catal. Today 2015, 244, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Carabineiro, S.A.C.; Konsolakis, M.; Marnellos, G.E.N.; Asad, M.F.; Soares, O.S.G.P.; Tavares, P.B.; Pereira, M.F.R.; De Melo Órfão, J.J.; Figueiredo, J.L. Ethyl acetate abatement on copper catalysts supported on ceria doped with rare earth oxides. Molecules 2016, 21, 644. [Google Scholar] [CrossRef] [Green Version]
- Díez-Ramírez, J.; Sánchez, P.; Kyriakou, V.; Zafeiratos, S.; Marnellos, G.E.; Konsolakis, M.; Dorado, F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J. CO2 Util. 2017, 21, 562–571. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Varvoutis, G.; Papista, E.; Marnellos, G.E. CO2 Hydrogenation over Nanoceria-Supported Transition Metal Catalysts: Role of Ceria Morphology (Nanorods versus Nanocubes) and Active Phase Nature (Co versus Cu). Nanomaterials 2019, 9, 1739. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Lykaki, M.; Papista, E.; Kaklidis, N.; Carabineiro, S.A.C.; Konsolakis, M. Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts 2019, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Stefa, S.; Lykaki, M.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Hydrothermal Synthesis of ZnO–doped Ceria Nanorods: Effect of ZnO Content on the Redox Properties and the CO Oxidation Performance. Appl. Sci. 2020, 10, 7605. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the preparation method on the physicochemical properties and the CO oxidation performance of nanostructured CeO2/TiO2 oxides. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Varvoutis, G.; Lykaki, M.; Stefa, S.; Papista, E.; Carabineiro, S.A.C.; Marnellos, G.E.; Konsolakis, M. Remarkable efficiency of Ni supported on hydrothermally synthesized CeO2 nanorods for low-temperature CO2 hydrogenation to methane. Catal. Commun. 2020, 142, 106036. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; Colussi, S.; de Leitenburg, C.; Trovarelli, A. Chapter 289—Ceria-Based Materials in Catalysis: Historical Perspective and Future Trends. Handb. Phys. Chem. Rare Earths 2016, 50, 209–242. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Volatile organic compounds abatement over copper-based catalysts: Effect of support. Inorg. Chim. Acta 2017, 455, 473–482. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Tavares, P.B.; Figueiredo, J.L. Redox properties and VOC oxidation activity of Cu catalysts supported on Ce1-xSmxOδ mixed oxides. J. Hazard. Mater. 2013, 261, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Ioakeimidis, Z. Surface/structure functionalization of copper-based catalysts by metal-support and/or metal-metal interactions. Appl. Surf. Sci. 2014, 320, 244–255. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Zhang, W.; Guo, S.; Su, H. Inverse rod-like CeO2 supported on CuO prepared by hydrothermal method for preferential oxidation of carbon monoxide. Catal. Commun. 2012, 23, 62–66. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Hrbek, J.; Evans, J.; Pérez, M. Water gas shift reaction on Cu and Au nanoparticles supported on CeO2(111) and ZnO(000): Intrinsic activity and importance of support interactions. Angew. Chem. Int. Ed. 2007, 46, 1329–1332. [Google Scholar] [CrossRef]
- Dong, L.; Yao, X.; Chen, Y. Interactions among supported copper-based catalyst components and their effects on performance: A review. Chin. J. Catal. 2013, 34, 851–864. [Google Scholar] [CrossRef]
- Pacchioni, G. Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Phys. Chem. Chem. Phys. 2013, 15, 1737. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Rodriguez, J.A.; Stacchiola, D. Electronic metal-support interactions and the production of hydrogen through the water-gas shift reaction and ethanol steam reforming: Fundamental studies with well-defined model catalysts. Top. Catal. 2013, 56, 1488–1498. [Google Scholar] [CrossRef]
- Capdevila-Cortada, M.; Vilé, G.; Teschner, D.; Pérez-Ramírez, J.; López, N. Reactivity descriptors for ceria in catalysis. Appl. Catal. B Environ. 2016, 197, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Mistry, H.; Behafarid, F.; Reske, R.; Varela, A.S.; Strasser, P.; Roldan Cuenya, B. Tuning Catalytic Selectivity at the Mesoscale via Interparticle Interactions. ACS Catal. 2016, 6, 1075–1080. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mistry, H.; Roldan Cuenya, B. Tailoring the Catalytic Properties of Metal Nanoparticles via Support Interactions. J. Phys. Chem. Lett. 2016, 7, 3519–3533. [Google Scholar] [CrossRef]
- Beckers, J.; Rothenberg, G. Sustainable selective oxidations using ceria-based materials. Green Chem. 2010, 12, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Razmgar, K.; Altarawneh, M.; Oluwoye, I.; Senanayake, G. Ceria-Based Catalysts for Selective Hydrogenation Reactions: A Critical Review. Catal. Surv. Asia 2021, 25, 27–47. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; De Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy Between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Effect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Zhang, H.; Andreescu, S.; Stanciu, L.A. CeO2–MOx (M: Zr, Ti, Cu) mixed metal oxides with enhanced oxygen storage capacity. J. Mater. Sci. 2015, 50, 3750–3762. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y. Catalysis based on nanocrystals with well-defined facets. Angew. Chem. Int. Ed. 2012, 51, 602–613. [Google Scholar] [CrossRef]
- Yuan, Q.; Duan, H.-H.; Li, L.-L.; Sun, L.-D.; Zhang, Y.-W.; Yan, C.-H. Controlled synthesis and assembly of ceria-based nanomaterials. J. Colloid Interface Sci. 2009, 335, 151–167. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Vilé, G.; Colussi, S.; Krumeich, F.; Trovarelli, A.; Pérez-Ramírez, J. Opposite face sensitivity of CeO2 in hydrogenation and oxidation catalysis. Angew. Chem. Int. Ed. 2014, 53, 12069–12072. [Google Scholar] [CrossRef]
- Ta, N.; Liu, J.; Shen, W. Tuning the shape of ceria nanomaterials for catalytic applications. Chin. J. Catal. 2013, 34, 838–850. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Caddeo, F.; Zhang, X.; Sakthivel, T.; Das, S.; Seal, S.; Ptasinska, S.; Sayle, D.C. Structure-Activity Map of Ceria Nanoparticles, Nanocubes, and Mesoporous Architectures. Chem. Mater. 2016, 28, 7287–7295. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Shen, W. Morphology-dependent nanocatalysis: Metal particles. Dalton Trans. 2011, 40, 5811–5826. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.T.; Nguyen, T.D.; Kleitz, F.; Do, T.O. Chapter 10–Shape-Controlled Synthesis of Metal Oxide Nanocrystals. In Book Controlled Nanofabrication: Advances and Applications, 1st ed.; Lui, R.-S., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2012; pp. 327–367. ISBN 978-981-4316-87-3. [Google Scholar]
- Datta, S.; Torrente-Murciano, L. Nanostructured faceted ceria as oxidation catalyst. Curr. Opin. Chem. Eng. 2018, 20, 99–106. [Google Scholar] [CrossRef]
- Roldan Cuenya, B. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; De Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Iliopoulou, E.; Carabineiro, S.A.C.; Konsolakis, M. Impact of the synthesis parameters on the solid state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv. 2017, 7, 6160–6169. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, B.; Tan, W.; Liu, B. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation. Catal. Commun. 2017, 95, 36–39. [Google Scholar] [CrossRef]
- Zawadzki, M. Preparation and characterization of ceria nanoparticles by microwave-assisted solvothermal process. J. Alloys Compd. 2008, 454, 347–351. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Si, R.; Liao, C.-S.; Yan, C.-H.; Xiao, C.-X.; Kou, Y. Facile alcohothermal synthesis, size-dependent ultraviolet absorption, and enhanced CO conversion activity of ceria nanocrystals. J. Phys. Chem. B 2003, 107, 10159–10167. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, D.; Cámara, A.L.; Monte, M.; Rasmussen, S.B.; Chinchilla, L.E.; Hungría, A.B.; Munuera, G.; Gyorffy, N.; Schay, Z.; Cortés Corberán, V.; et al. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Characterization and performance as a function of the exposed face present in the CeO2 support. Appl. Catal. B Environ. 2013, 130–131, 224–238. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured Ceria-Based Materials: Effect of the Hydrothermal Synthesis Conditions on the Structural Properties and Catalytic Activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Partial oxidation of methanol over copper supported on nanoshaped ceria for hydrogen production. Catal. Today 2017, 282, 185–194. [Google Scholar] [CrossRef]
- Yao, S.Y.; Xu, W.Q.; Johnston-Peck, A.C.; Zhao, F.Z.; Liu, Z.Y.; Luo, S.; Senanayake, S.D.; Martínez-Arias, A.; Liu, W.J.; Rodriguez, J.A. Morphological effects of the nanostructured ceria support on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction. Phys. Chem. Chem. Phys. 2014, 16, 17183–17195. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martínez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D.; et al. In Situ Characterization of Cu/CeO2 Nanocatalysts for CO2 Hydrogenation: Morphological Effects of Nanostructured Ceria on the Catalytic Activity. J. Phys. Chem. C 2018, 122, 12934–12943. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: Insight into catalytic activity and sintering. Appl. Catal. B Environ. 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. Effect of the morphological and surface properties of CeO2-based catalysts on the soot oxidation activity. Chem. Eng. J. 2015, 278, 190–198. [Google Scholar] [CrossRef]
- Gawade, P.; Mirkelamoglu, B.; Ozkan, U.S. The role of support morphology and impregnation medium on the water gas shift activity of ceria-supported copper catalysts. J. Phys. Chem. C 2010, 114, 18173–18181. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- He, H.-W.; Wu, X.-Q.; Ren, W.; Shi, P.; Yao, X.; Song, Z.-T. Synthesis of crystalline cerium dioxide hydrosol by a sol-gel method. Ceram. Int. 2012, 38 (Suppl. 1), S501–S504. [Google Scholar] [CrossRef]
- Phonthammachai, N.; Rumruangwong, M.; Gulari, E.; Jamieson, A.M.; Jitkarnka, S.; Wongkasemjit, S. Synthesis and rheological properties of mesoporous nanocrystalline CeO2 via sol-gel process. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 61–68. [Google Scholar] [CrossRef]
- Pinjari, D.V.; Pandit, A.B. Room temperature synthesis of crystalline CeO2 nanopowder: Advantage of sonochemical method over conventional method. Ultrason. Sonochem. 2011, 18, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, Y.; Pang, G.; Koltypin, Y.; Gedanken, A. Sonochemical synthesis of cerium oxide nanoparticles—Effect of additives and quantum size effect. J. Colloid Interface Sci. 2002, 246, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zhang, X.; Xu, J.; Han, Y. Effects of preparation methods on the activity of CuO/CeO2 catalysts for CO oxidation. Front. Chem. Sci. Eng. 2017, 11, 603–612. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-W.; Yu, W.-Z.; Du, P.-P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.-J.; Yan, C.-H. Crystal Plane Effect of Ceria on Supported Copper Oxide Cluster Catalyst for CO Oxidation: Importance of Metal-Support Interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ohshima, T.; Tezuka, Y.; Katayama, M.; Katoh, M.; Sugiyama, S. Morphological effects of CeO2 nanostructures for catalytic soot combustion of CuO/CeO2. Catal. Today 2015, 246, 67–71. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, P.; Kumar, R.; Mehta, S.K. Nanoscale surface designing of Cerium oxide nanoparticles for controlling growth, stability, optical and thermal properties. Ceram. Int. 2015, 41, 10995–11003. [Google Scholar] [CrossRef]
- Miyazaki, H.; Kato, J.I.; Sakamoto, N.; Wakiya, N.; Ota, T.; Suzuki, H. Synthesis of CeO2 nanoparticles by rapid thermal decomposition using microwave heating. Adv. Appl. Ceram. 2010, 109, 123–127. [Google Scholar] [CrossRef]
- Yang, H.; Huang, C.; Tang, A.; Zhang, X.; Yang, W. Microwave-assisted synthesis of ceria nanoparticles. Mater. Res. Bull. 2005, 40, 1690–1695. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Reactivity of methanol over copper supported on well-shaped CeO2: A TPD-DRIFTS study. Catal. Sci. Technol. 2017, 7, 5224–5235. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Hillary, B.; Amin, M.H.; Rockstroh, N.; Bentrup, U.; Brückner, A.; Bhargava, S.K. Heterostructured Copper-Ceria and Iron-Ceria Nanorods: Role of Morphology, Redox, and Acid Properties in Catalytic Diesel Soot Combustion. Langmuir 2018, 34, 2663–2673. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Kovacevic, M.; Mojet, B.L.; Van Ommen, J.G.; Lefferts, L. Effects of Morphology of Cerium Oxide Catalysts for Reverse Water Gas Shift Reaction. Catal. Lett. 2016, 146, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Piumetti, M.; Andana, T.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Study on the CO Oxidation over Ceria-Based Nanocatalysts. Nanoscale Res. Lett. 2016, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Mock, S.A.; Sharp, S.E.; Stoner, T.R.; Radetic, M.J.; Zell, E.T.; Wang, R. CeO2 nanorods-supported transition metal catalysts for CO oxidation. J. Colloid Interface Sci. 2016, 466, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-dependent properties of Cu/CeO2 catalysts for the water-gas shift reaction. Catalysts 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Yang, P.; Li, J.; Shi, R.; Chen, L.; Zhang, A.; Zhu, Y. Controllable synthesis, characterization, and CO oxidation activity of CeO2 nanostructures with various morphologies. Ceram. Int. 2016, 42, 7810–7818. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Cui, Y.; Dai, W.-L. Support morphology and crystal plane effect of Cu/CeO2 nanomaterial on the physicochemical and catalytic properties for carbonate hydrogenation. Catal. Sci. Technol. 2016, 6, 7752–7762. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Deng, Y.; Gao, F.; Liu, B.; Dong, L. Morphology and Crystal-Plane Effects of Nanoscale Ceria on the Activity of CuO/CeO2 for NO Reduction by CO. ChemCatChem 2011, 3, 978–989. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Jing, G.; Zhang, H.; Zeng, S.; Tian, X.; Zou, X.; Wen, J.; Su, H.; Zhong, C.-J.; et al. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes. Appl. Catal. B Environ. 2018, 239, 665–676. [Google Scholar] [CrossRef]
- Roldan Cuenya, B.; Behafarid, F. Nanocatalysis: Size- and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 2015, 70, 135–187. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Tian, Y.; Ding, T.; Zheng, L.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, L.; Lu, J.; Chen, R.; Lei, Y.; Chen, K.; Han, C.; He, S.; Wan, G.; Luo, Y. A solvent-free method to rapidly synthesize CuO-CeO2 catalysts to enhance their CO preferential oxidation: Effects of Cu loading and calcination temperature. Mol. Catal. 2017, 443, 241–252. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bastos, S.S.T.; Órfão, J.J.M.; Pereira, M.F.R.; Delgado, J.J.; Figueiredo, J.L. Exotemplated ceria catalysts with gold for CO oxidation. Appl. Catal. A Gen. 2010, 381, 150–160. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Silva, A.M.T.; Dražić, G.; Tavares, P.B.; Figueiredo, J.L. Gold nanoparticles on ceria supports for the oxidation of carbon monoxide. Catal. Today 2010, 154, 21–30. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M., III; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Solsona, B.; Sanchis, R.; Dejoz, A.M.; García, T.; Ruiz-Rodríguez, L.; López Nieto, J.M.; Cecilia, J.A.; Rodríguez-Castellón, E. Total Oxidation of Propane Using CeO2 and CuO-CeO2 Catalysts Prepared Using Templates of Different Nature. Catalysts 2017, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Qiu, N.; Zhang, J.; Wu, Z. Peculiar surface-interface properties of nanocrystalline ceria-cobalt oxides with enhanced oxygen storage capacity. Phys. Chem. Chem. Phys. 2014, 16, 22659–22664. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Agostinho Moreira, J.; Romaguera-Barcelay, Y.; Figueiredo, J.L. Effect of preparation method on the solid state properties and the deN2O performance of CuO-CeO2 oxides. Catal. Sci. Technol. 2015, 5, 3714–3727. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Sayle, T.X.T.; Cantoni, M.; Bhatta, U.M.; Parker, S.C.; Hall, S.R.; Möbus, G.; Molinari, M.; Reid, D.; Seal, S.; Sayle, D.C. Strain and architecture-tuned reactivity in ceria nanostructures; Enhanced catalytic oxidation of CO to CO2. Chem. Mater. 2012, 24, 1811–1821. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen defects and surface chemistry of ceria: Quantum chemical studies compared to experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Gao, F.; Kovarik, L.; Peden, C.H.F.; Wang, Y. Effects of CeO2 support facets on VOx/CeO2 catalysts in oxidative dehydrogenation of methanol. J. Catal. 2014, 315, 15–24. [Google Scholar] [CrossRef]
- Mudiyanselage, K.; Senanayake, S.D.; Feria, L.; Kundu, S.; Baber, A.E.; Graciani, J.; Vidal, A.B.; Agnoli, S.; Evans, J.; Chang, R.; et al. Importance of the metal-oxide interface in catalysis: In situ studies of the water-gas shift reaction by ambient-pressure X-ray photoelectron spectroscopy. Angew. Chem. Int. Ed. 2013, 52, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Mayernick, A.D.; Janik, M.J. Methane activation and oxygen vacancy formation over CeO2 and Zr, Pd substituted CeO2 surfaces. J. Phys. Chem. C 2008, 112, 14955–14964. [Google Scholar] [CrossRef]
- Nolan, M.; Grigoleit, S.; Sayle, D.C.; Parker, S.C.; Watson, G.W. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria. Surf. Sci. 2005, 576, 217–229. [Google Scholar] [CrossRef]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, R.; Sun, X.; Chen, H.; Tian, Q.; Shen, D.; Li, Y. Favorable synergetic effects between CuO and the reactive planes of ceria nanorods. Catal. Lett. 2005, 101, 169–173. [Google Scholar] [CrossRef]
- Dong, F.; Meng, Y.; Han, W.; Zhao, H.; Tang, Z. Morphology effects on surface chemical properties and lattice defects of Cu/CeO2 catalysts applied for low-temperature CO oxidation. Sci. Rep. 2019, 9, 12056. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, Z.; Li, Z.; Huang, W. Understanding morphology-dependent CuOx-CeO2 interactions from the very beginning. Chin. J. Catal. 2020, 41, 1006–1016. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Wu, L.; Qin, W.; Hu, X.; Ju, S.; Dong, C.; Yang, Y. Decomposition and reduction of N2O on CaS (100) surface: A theoretical account. Surf. Sci. 2015, 632, 83–87. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R. CO2 recycling by reaction with renewably-generated hydrogen. Int. J. Greenh. Gas Control 2010, 4, 44–50. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R. Sustainable technologies for the reclamation of greenhouse gas CO2. J. Clean. Prod. 2015, 103, 784–792. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Kacimi, S.; Barbier, J., Jr.; Taha, R.; Duprez, D. Oxygen storage capacity of promoted Rh/CeO2 catalysts. Exceptional behavior of RhCu/CeO2. Catal. Lett. 1993, 22, 343–350. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrogen Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Martin, N.M.; Velin, P.; Skoglundh, M.; Bauer, M.; Carlsson, P.-A. Catalytic hydrogenation of CO2 to methane over supported Pd, Rh and Ni catalysts. Catal. Sci. Technol. 2017, 7, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ. 2012, 113–114, 237–249. [Google Scholar] [CrossRef]
- Dreyer, J.A.H.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.H.-L.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru-based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the Influence of CeO2 Crystal Facet on CO2 Hydrogenation to Methanol over Pd/CeO2 Catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 hydrogenation over differently morphological CeO2-supported Cu-Ni catalysts. Int. J. Energy Res. 2019, 43, 5392–5404. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 Hydrogenation to Methanol over a Highly Active Cu-Ni/CeO2-Nanotube Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Pérez, V.R.; Bueno-López, A. Catalytic regeneration of Diesel Particulate Filters: Comparison of Pt and CePr active phases. Chem. Eng. J. 2015, 279, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic diesel particulate filters with highly dispersed ceria: Effect of the soot-catalyst contact on the regeneration performance. Appl. Catal. B Environ. 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Lv, C.; Chen, H.; Hu, M.; Ai, T.; Fu, H. Nano-oxides washcoat for enhanced catalytic oxidation activity toward the perovskite-based monolithic catalyst. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]














| Adjusted Parameter | Main Implications in Physicοchemical Properties |
|---|---|
| Composition (e.g., binary or ternary MOs) | surface area; structural characteristics |
| Size (e.g., metal particles of nanometer size) | surface area; electronic environment; coordination environment; structural defects |
| Shape (e.g., nanorods and nanocubes) | energy formation of anionic vacancies; redox features |
| Electronic state (e.g., alkali addition) | work function; redox characteristics |
| Chemical state (e.g., incorporation of rGO) | redox properties; structural defects; electronic environment |
| BET Analysis | XRD Analysis | TPR Analysis | ||
|---|---|---|---|---|
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Crystallite Diameter, DXRD (nm) 1 | OSC (mmol O2/g) 2 |
| CeO2-NC | 40 | 0.12 | 19.2 | 0.21 |
| CeO2-NR | 92 | 0.71 | 13.2 | 0.29 |
| CeO2-NP | 109 | 1.04 | 9.5 | 0.24 |
| Samples | XPS Analysis | |
|---|---|---|
| OI/OII | Ce3+/Ce4+ | |
| CeO2-NC | 1.99 | 0.33 |
| CeO2-NP | 2.04 | 0.32 |
| CeO2-NR | 2.13 | 0.33 |
| Sample | BET Analysis | XRD Analysis | TPR Analysis | ||
|---|---|---|---|---|---|
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Crystallite Diameter, DXRD (nm) | OSC (mmol O2/g) | ||
| CeO2 | CuO/Co3O4/Fe2O3/NiO | ||||
| Cu/CeO2-NC | 34.3 | 0.29 | 19.2 | 52 | 0.75 |
| Cu/CeO2-NR | 75.4 | 0.40 | 11.6 | 43 | 0.90 |
| Cu/CeO2-NP | 90.7 | 0.29 | 9.6 | 31 | 0.83 |
| Co/CeO2-NC | 27.9 | 0.15 | 24 | 19 | 1.03 |
| Co/CeO2-NR | 71.8 | 0.31 | 14 | 16 | 1.19 |
| Co/CeO2-NP | 70.5 | 0.17 | 11 | 15 | 1.20 |
| Fe/CeO2-NC | 32.2 | 0.19 | 16.8 | 52.3 | 0.67 |
| Fe/CeO2-NR | 68.6 | 0.19 | 9.7 | 7.2 | 0.75 |
| Fe/CeO2-NP | 64.2 | 0.12 | 8.5 | 16.5 | 0.70 |
| Ni/CeO2-NC | 31.8 | 0.21 | 22 | 16.5 | 0.78 |
| Ni/CeO2-NR | 72.0 | 0.38 | 14 | 23 | 0.92 |
| Ni/CeO2-NP | 73.0 | 0.27 | 12 | 23 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konsolakis, M.; Lykaki, M. Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review. Catalysts 2021, 11, 452. https://doi.org/10.3390/catal11040452
Konsolakis M, Lykaki M. Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review. Catalysts. 2021; 11(4):452. https://doi.org/10.3390/catal11040452
Chicago/Turabian StyleKonsolakis, Michalis, and Maria Lykaki. 2021. "Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review" Catalysts 11, no. 4: 452. https://doi.org/10.3390/catal11040452
APA StyleKonsolakis, M., & Lykaki, M. (2021). Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified by CeO2-Based Transition Metal Catalysts: A Critical Review. Catalysts, 11(4), 452. https://doi.org/10.3390/catal11040452