Molecular Cage Promoted Aerobic Oxidation or Photo-Induced Rearrangement of Spiroepoxy Naphthalenone
Abstract
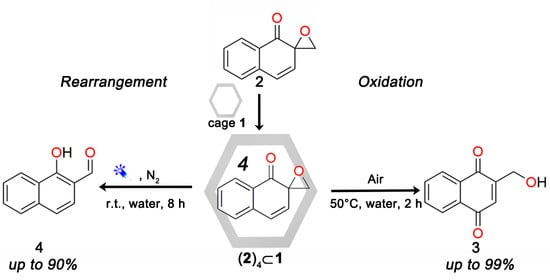
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Preparation and Catalysis Procedure
4.3. Computational Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Fujita, D.; Ueda, Y.; Sato, S.; Yokoyama, H.; Mizuno, N.; Kumasaka, T.; Fujita, M. Self-Assembly of M 30 L 60 Icosidodecahedron. Chem 2016, 1, 91–101. [Google Scholar] [CrossRef]
- Yang, D.; Greenfield, J.L.; Ronson, T.K.; Von Krbek, L.K.S.; Yu, L.; Nitschke, J.R. LaIII and ZnII Cooperatively Template a Metal–Organic Capsule. J. Am. Chem. Soc. 2020, 142, 19856–19861. [Google Scholar] [CrossRef]
- Cheng, P.; Cai, L.; Li, S.; Hu, S.; Yan, D.; Zhou, L.; Sun, Q. Guest-Reaction Driven Cage to Conjoined Twin-Cage Mitosis-Like Host Transformation. Angew. Chem. Int. Ed. 2020, 59, 23569–23573. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Song, B.; Zhang, Y.; Jiang, X.; Wang, M.; Trumbleson, R.; Liu, C.; Wang, P.; Hao, X.-Q.; et al. Intra- and intermolecular self-assembly of a 20-nm-wide supramolecular hexagonal grid. Nat. Chem. 2020, 12, 468–474. [Google Scholar] [CrossRef]
- Cai, L.-X.; Yan, D.-N.; Cheng, P.-M.; Xuan, J.-J.; Li, S.-C.; Zhou, L.-P.; Tian, C.-B.; Sun, Q.-F. Controlled Self-Assembly and Multistimuli-Responsive Interconversions of Three Conjoined Twin-Cages. J. Am. Chem. Soc. 2021, 143, 2016–2024. [Google Scholar] [CrossRef]
- Liu, D.; Li, K.; Chen, M.; Zhang, T.; Li, Z.; Yin, J.-F.; He, L.; Wang, J.; Yin, P.; Chan, Y.-T.; et al. Russian-Doll-Like Molecular Cubes. J. Am. Chem. Soc. 2021, 143, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiao, Z.; Li, J.; Lollar, C.; Liu, L.; Lian, X.; Yuan, S.; Banerjee, S.; Zhang, P.; Zhou, H.-C. Formation of a Highly Reactive Cobalt Nanocluster Crystal within a Highly Negatively Charged Porous Coordination Cage. Angew. Chem. Int. Ed. 2018, 57, 5283–5287. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Lu, W.; Luo, D.; Zhu, X.-W.; Li, X.; Zhou, X.-P.; Li, D. Fine-Tuning Apertures of Metal–Organic Cages: Encapsulation of Carbon Dioxide in Solution and Solid State. J. Am. Chem. Soc. 2019, 141, 11621–11627. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Shi, X.; Phan, H.; Qu, H.; Hu, Y.-X.; Yin, G.-Q.; Zhao, X.-L.; Li, X.; Xu, L.; Yu, Q.; et al. Efficient self-assembly of heterometallic triangular necklace with strong antibacterial activity. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-X.; Feng, H.-J.; Guo, B.-B.; Lu, Y.; Jin, G.-X. Coordination-Directed Construction of Molecular Links. Chem. Rev. 2020, 120, 6288–6325. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, D.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Self-assembly of metalla[3]catenanes, borromean rings and ring-in-ring complex using a simple π-donor unit. Natl. Sci. Rev. 2020, 7, 1548–1556. [Google Scholar] [CrossRef]
- Huang, S.-L.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Self-Assembly of Molecular Borromean Rings from Bimetallic Coordination Rectangles. Angew. Chem. Int. Ed. 2014, 53, 11218–11222. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional Molecular Flasks: New Properties and Reactions within Discrete, Self-Assembled Hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chu, D.; Tang, X.; Liu, Y.; Xuan, W.; Cui, Y. Supramolecular Coordination Cages for Asymmetric Catalysis. Chem. A Eur. J. 2019, 25, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wu, K.; Zhang, J.-H.; Su, C.-Y. Chiral metal–organic cages/containers (MOCs): From structural and stereochemical design to applications. Coord. Chem. Rev. 2019, 378, 333–349. [Google Scholar] [CrossRef]
- Zhao, L.; Jing, X.; Li, X.; Guo, X.; Zeng, L.; He, C.; Duan, C. Catalytic properties of chemical transformation within the confined pockets of Werner-type capsules. Coord. Chem. Rev. 2019, 378, 151–187. [Google Scholar] [CrossRef]
- Morimoto, M.; Bierschenk, S.M.; Xia, K.T.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Advances in supramolecular host-mediated reactivity. Nat. Catal. 2020, 3, 969–984. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Merget, S.; Catti, L.; Piccini, G.; Tiefenbacher, K. Requirements for Terpene Cyclizations inside the Supramolecular Resorcinarene Capsule: Bound Water and Its Protonation Determine the Catalytic Activity. J. Am. Chem. Soc. 2020, 142, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Enzymelike Catalysis of the Nazarov Cyclization by Supramolecular Encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastings, C.J.; Fiedler, D.; Bergman, R.G.; Raymond, K.N. Aza Cope Rearrangement of Propargyl Enammonium Cations Catalyzed by a Self-Assembled “Nanozyme”. J. Am. Chem. Soc. 2008, 130, 10977–10983. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.M.; Morimoto, M.; Kapustin, E.A.; Alzakhem, N.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Deconvoluting the Role of Charge in a Supramolecular Catalyst. J. Am. Chem. Soc. 2018, 140, 6591–6595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Cai, J.; Li, Y.; Wei, J.; Duan, C. A host–guest approach to combining enzymatic and artificial catalysis for catalyzing biomimetic monooxygenation. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Guo, J.; Fan, Y.; Lu, Y.; Zheng, S.; Su, C. Visible-Light Photocatalysis of Asymmetric [2+2] Cycloaddition in Cage-Confined Nanospace Merging Chirality with Triplet-State Photosensitization. Angew. Chem. Int. Ed. 2020, 59, 8661–8669. [Google Scholar] [CrossRef]
- Wang, J.-S.; Wu, K.; Yin, C.; Li, K.; Huang, Y.; Ruan, J.; Feng, X.; Hu, P.; Su, C.-Y. Cage-confined photocatalysis for wide-scope unusually selective [2 + 2] cycloaddition through visible-light triplet sensitization. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Zaffaroni, R.; Orth, N.; Ivanović-Burmazović, I.; Reek, J.N.H. Hydrogenase Mimics in M 12 L 24 Nanospheres to Control Overpotential and Activity in Proton-Reduction Catalysis. Angew. Chem. Int. Ed. 2020, 59, 18485–18489. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Wang, X.-Z.; Dai, R.-R.; Huang, Y.-L.; Zhou, X.-C.; Zhou, X.-P.; Li, D. Self-assembly of mixed-valence and heterometallic metallocycles: Efficient catalysts for the oxidation of alcohols to aldehydes in ambient air. Dalton Trans. 2020, 49, 7304–7308. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.-W.; Luo, W.; Zhou, J.-Y.; Ma, X.-J.; Chen, G.-H.; Zhou, X.-P.; Li, D. Hydroxo Iron(III) Sites in a Metal–Organic Framework: Proton-Coupled Electron Transfer and Catalytic Oxidation of Alcohol with Molecular Oxygen. ACS Appl. Mater. Interfaces 2019, 11, 45621–45628. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, J.; Togo, T.; Jin, F.; Xiao, Z.; Liu, L.; Drake, H.; Lian, X.; Zhou, H.-C. Ultra-Small Face-Centered-Cubic Ru Nanoparticles Confined within a Porous Coordination Cage for Dehydrogenation. Chem 2018, 4, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.-L.; Shen, J.-C.; Peng, Z.; Wu, G.-Y.; Yin, G.-Q.; Shi, X.; Yang, H.-B. Controllable synthesis of ultrasmall Pd nanocatalysts templated by supramolecular coordination cages for highly efficient reductive dehalogenation. J. Mater. Chem. A 2020, 8, 12097–12105. [Google Scholar] [CrossRef]
- Wang, J.; Young, T.A.; Duarte, F.; Lusby, P.J. Synergistic Noncovalent Catalysis Facilitates Base-Free Michael Addition. J. Am. Chem. Soc. 2020, 142, 17743–17750. [Google Scholar] [CrossRef]
- Zhu, F.-F.; Chen, L.-J.; Chen, S.; Wu, G.-Y.; Jiang, W.-L.; Shen, J.-C.; Qin, Y.; Xu, L.; Yang, H.-B. Confinement Self-Assembly of Metal-Organic Cages within Mesoporous Carbon for One-Pot Sequential Reactions. Chem 2020, 6, 2395–2406. [Google Scholar] [CrossRef]
- Cullen, W.; Misuraca, M.C.; Hunter, M.C.M.C.A.; Williams, N.H.; Ward, W.C.N.H.W.M.D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ngai, C.; Sanchez-Marsetti, C.M.; Harman, W.H.; Hooley, R.J. Supramolecular Catalysis of the oxa-Pictet–Spengler Reaction with an Endohedrally Functionalized Self-Assembled Cage Complex. Angew. Chem. Int. Ed. 2020, 59, 23505–23509. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, H.; Shitozawa, K.; Fujita, M. Enhanced reactivity of twisted amides inside a molecular cage. Nat. Chem. 2020, 12, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Châtelet, B.; Dufaud, V.; Hérault, D.; Michaud-Chevallier, S.; Robert, V.; Dutasta, J.-P.; Martinez, A. Endohedral Functionalized Cage as a Tool to Create Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2018, 57, 14212–14215. [Google Scholar] [CrossRef]
- Cai, L.-X.; Li, S.-C.; Yan, D.-N.; Zhou, L.-P.; Guo, F.; Sun, Q.-F. Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis. J. Am. Chem. Soc. 2018, 140, 4869–4876. [Google Scholar] [CrossRef]
- Li, S.-C.; Cai, L.-X.; Zhou, L.-P.; Guo, F.; Sun, Q.-F. Supramolecular synthesis of coumarin derivatives catalyzed by a coordination-assembled cage in aqueous solution. Sci. China Ser. B Chem. 2019, 62, 713–718. [Google Scholar] [CrossRef]
- Gesson, J.-P.; Mondon, M.; Pokrovska, N. Synthesis of Fused Aromatic [1,3]Dioxoles from 2-Hydroxymethylphenols. Synlett 1997, 12, 1395–1396. [Google Scholar] [CrossRef]
- He, J.; Ling, J.; Chiu, P. Vinyl Epoxides in Organic Synthesis. Chem. Rev. 2014, 114, 8037–8128. [Google Scholar] [CrossRef]
- Xin, M.; Bugg, T.D.H. Biomimetic Formation of 2-Tropolones by Dioxygenase-Catalysed Ring Expansion of Substituted 2,4-Cyclohexadienones. ChemBioChem 2009, 11, 272–276. [Google Scholar] [CrossRef]
- Tius, M.A.; Reddy, N.K. Stereoselective Synthesis of Disubstituted Naphthalene-1,2-oxides. Synth. Commun. 1994, 24, 859–869. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Sato, N.; Fujita, M. Selective Enclathration of Linear Alkanols by a Self-assembled Coordination Cage. Application to the Catalytic Wacker Oxidation of ω-Alkenols. Chem. Lett. 2005, 34, 1392–1393. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Gurbanov, A.V.; Guseinov, F.I.; da Silva, M.F.C.G. Noncovalent interactions in metal complex catalysis. Coord. Chem. Rev. 2019, 387, 32–46. [Google Scholar] [CrossRef]
- Ma, Z.; Mahmudov, K.T.; Aliyeva, V.A.; Gurbanov, A.V.; Pombeiro, A.J. TEMPO in metal complex catalysis. Coord. Chem. Rev. 2020, 423, 213482. [Google Scholar] [CrossRef]
- Ma, Z.; Mahmudov, K.T.; Aliyeva, V.A.; Gurbanov, A.V.; da Silva, M.F.C.G.; Pombeiro, A.J. Peroxides in metal complex catalysis. Coord. Chem. Rev. 2021, 437, 213859. [Google Scholar] [CrossRef]
- Higuchi, M.; Yamaguchi, S.; Hirao, T. Construction of Palladium-Polypyrrole Catalytic System in the Wacker Oxidation. Synlett 1996, 1996, 1213–1214. [Google Scholar] [CrossRef]
- Mitsudome, T.; Umetani, T.; Nosaka, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Convenient and Efficient Pd-Catalyzed Regioselective Oxyfunctionalization of Terminal Olefins by Using Molecular Oxygen as Sole Reoxidant. Angew. Chem. Int. Ed. 2006, 45, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.-D.; Bremholt, T.; Adler, E. Oxidative formation and photochemical isomerization of spiro-epoxy-2,4-cyclohexadienones. Tetrahedron Lett. 1972, 13, 4205–4208. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]




 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Conditions | LEDs | Time (h) | Solvent | Yield a | ||
| 3 | 4 | |||||||
| 1 | 1 | Air | 50 °C | - | 2 h | D2O | >99% | - |
| 2 | - | Air | 50 °C | - | 2 h | H2O | - | - |
| 3 | 1 | N2 | 50 °C | - | 2 h | D2O | - | - |
| 4 b | L | Air | 50 °C | - | 2 h | H2O | - | - |
| 5 c | Pd | Air | 50 °C | - | 2 h | H2O | 39% | - |
| 6 | Ph4B⊂1 | Air | 50 °C | - | 2 h | D2O | 36% | - |
| 7 d | 1 | Air | 50 °C | - | 4 h | D2O | 95% | - |
| 8 | 1 | N2 | r.t. | blue | 8 h | D2O | - | 90% |
| 9 | - | Air | r.t. | blue | 8 h | H2O | - | 32% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Cai, L.; Yan, D.; Zhou, L.; Sun, Q. Molecular Cage Promoted Aerobic Oxidation or Photo-Induced Rearrangement of Spiroepoxy Naphthalenone. Catalysts 2021, 11, 484. https://doi.org/10.3390/catal11040484
Cheng P, Cai L, Yan D, Zhou L, Sun Q. Molecular Cage Promoted Aerobic Oxidation or Photo-Induced Rearrangement of Spiroepoxy Naphthalenone. Catalysts. 2021; 11(4):484. https://doi.org/10.3390/catal11040484
Chicago/Turabian StyleCheng, Peiming, Lixuan Cai, Danni Yan, Lipeng Zhou, and Qingfu Sun. 2021. "Molecular Cage Promoted Aerobic Oxidation or Photo-Induced Rearrangement of Spiroepoxy Naphthalenone" Catalysts 11, no. 4: 484. https://doi.org/10.3390/catal11040484
APA StyleCheng, P., Cai, L., Yan, D., Zhou, L., & Sun, Q. (2021). Molecular Cage Promoted Aerobic Oxidation or Photo-Induced Rearrangement of Spiroepoxy Naphthalenone. Catalysts, 11(4), 484. https://doi.org/10.3390/catal11040484