Polyaniline/Ag2S–CdS Nanocomposites as Efficient Electrocatalysts for Triiodide Reduction in Dye-Sensitized Solar Cells
Abstract
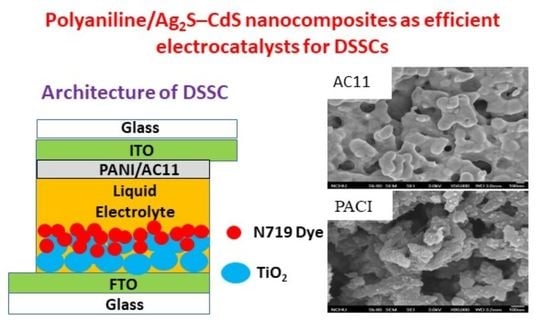
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ag2S–CdS Nanocomposite
2.2. Characterization of PANI/Ag2S–CdS Nanocomposites
2.3. Morphologies and Electrochemical and PV Properties of PANI/Ag2S–CdS Composite-Based Counter Electrodes
3. Materials and Methods
3.1. Chemicals
3.2. Ag2S–CdS Nanocomposite
3.2.1. CdS NPs
3.2.2. Ag2S
3.2.3. Ag2S–CdS Nanocomposite AC11
3.3. PANI/Ag2S–CdS Nanocomposites
3.3.1. PANI
3.3.2. PACI
3.3.3. PACO
3.4. Counter Electrodes
3.4.1. PANI-Based Electrodes (PANI-S)
3.4.2. PACI-Based Electrodes (PACI-S)
3.4.3. PACO-Based Electrodes (PACO-S)
3.5. DSSCs
3.6. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Baker, R.H.; Muller, E.; Liska, P.; Vlachopoulos, N.; Gratzel, M. Conversion of Light to Electricity by cis-X2bis(2,2´-bipyridyl-4,4´-dicarboxylate)ruthenium(II) Charge-transfer Sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Angelis, F.D.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Gratzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.K.; Wang, R.H.; Wu, J.L.; Jyothibasu, J.P.; Wang, T.L.; Chu, C.Y.; Lee, R.H. Synthesis of a Series of Novel Imidazolium-containing Ionic Liquid Copolymers for Dye-sensitized Solar Cells. Polymer 2020, 210, 123074. [Google Scholar] [CrossRef]
- Cai, C.Y.; Tseng, S.K.; Kuo, M.; Lin, K.Y.A.; Yang, H.; Lee, R.H. Photovoltaic Performance of a N719 Dye Based Dye-sensitized Solar Cell with Transparent Macroporous Anti-ultraviolet Photonic Crystal Coatings. RSC Adv. 2015, 5, 102803–102810. [Google Scholar] [CrossRef]
- Lee, R.H.; Chi, C.H.; Hsu, Y.C. Platinum Nanoparticle/Self-doping Polyaniline Composite Based Counter Electrodes for Dye-sensitized Solar Cells. J. Nanopart. Res. 2013, 15, 1733. [Google Scholar] [CrossRef]
- Karakuş, M.O.; Yakışıklıer, M.E.; Delibaş, A.; Ayyıldız, E.; Çetin, H. Anionic and Cationic Polymer-based Quasi-solid-state Dye-sensitized Solar Cell with Poly(aniline) Counter Electrode. Sol. Energy 2020, 195, 565–572. [Google Scholar] [CrossRef]
- Bumika, M.; Mallick, M.K.; Palai, A.K.; Mohanty, S.; Nayak, S.K. Electrosynthesis of Polyaniline-based Composite Films and Their Electrochemical Activity. J. Appl. Polym. Sci. 2021, 138, e49827. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Tseng, L.C.; Lee, R.H. Graphene Oxide Sheet–polyaniline Nanohybrids for Enhanced Photovoltaic Performance of Dye-sensitized Solar Cells. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 321. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chen, G.L.; Lee, R.H. Graphene Oxide Sheet-polyaniline Nanocomposite Prepared Through in situ Polymerization/Deposition Method for Counter Electrode of Dye-sensitized Solar Cell. J. Polym. Res. 2014, 21, 440. [Google Scholar] [CrossRef]
- Sudhakar, V.; Singh, A.K.; Chini, M.K. Nanoporous Reduced Graphene Oxide and Polymer Composites as Efficient Counter Electrodes in Dye-sensitized Solar Cells. ACS Appl. Electron. Mater. 2020, 2, 626–634. [Google Scholar] [CrossRef]
- Wahyuono, R.A.; Jia, G.; Plentz, J.; Dellith, A.; Dellith, J.; Westendorf, F.H.; Seyring, M.; Presselt, M.; Andra, G.; Rettenmayr, M.; et al. Self-assembled Graphene/MWCNT Bilayers as Platinum-free Counter Electrode in Dye-sensitized Solar Cells. Chem. Phys. Chem. 2019, 20, 3336–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samantaray, M.R. , Mondal, A.K.; Murugadoss, G.; Pitchaimuthu, S.; Das, S.; Bahru, R.; Mohamed, M.A. Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-sensitized Solar Cells: A Review. Materials 2020, 13, 2779. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Yang, Q.; Yang, H.; Zhou, Y. Compacted Stainless Steel Mesh-supported Co3O4 Porous Nanobelts for HCHO Catalytic Oxidation and Co3O4@Co3S4 via in situ Sulfurization as Platinum-free Counter Electrode for Flexible Dye-sensitized Solar Cells. Appl. Surf. Sci. 2021, 536, 147815. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Sonar, P.; Jain, S.M.; Jeong, S.K.; Grace, A.N. Performance Evaluation of a Low-cost, Novel Vanadium Nitride Xerogel (VNXG) as a Platinum-free Electrocatalyst for Dye-sensitized Solar Cells. RSC Adv. 2020, 10, 41177–41186. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, Y.J.; Li, C.T.; Lee, C.P.; Lin, J.T.; Ho, K.C. Boron Nitride/Sulfonated Polythiophene Composite Electrocatalyst as the TCO and Pt-free Counter Electrode for Dye-sensitized Solar Cells: 21% at Dim Light. ACS Sustain. Chem. Eng. 2020, 8, 5251–5259. [Google Scholar] [CrossRef]
- Majid, A.; Ullah, I.; Kubra, K.T.; Khan, S.U.D.; Haider, S. First Principles Study of Transition Metals Doped SiC for Application as Counter Electrode in DSSC. Surf. Sci. 2019, 687, 41–47. [Google Scholar] [CrossRef]
- Zhong, J.; Peng, Y.; Zhou, M.; Zhao, J.; Liang, S.; Wang, H.; Cheng, Y.B. Facile Synthesis of Nanoporous TiC–SiC–C Composites as a Novel Counter-electrode for Dye Sensitized Solar Cells. Micropor. Mesopor. Mater. 2014, 190, 309–315. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Meenakshamma, A.; Siddeswaramma, G.; Susmitha, K.; Subbaiah, Y.P.V.; Narayana, T.; Raghavender, M. Electrodeposited MoS2 Counter Electrode for Flexible Dye Sensitized Solar Cell Module with Ionic Liquid Assisted Photoelectrode. Sol. Energy 2020, 199, 447–452. [Google Scholar] [CrossRef]
- Hussain, S.; Patil, S.A.; Vikraman, D.; Rabani, I.; Arbab, A.A.; Jeong, S.H.; Kim, H.S.; Choi, H.; Jung, J. Enhanced Electrocatalytic Properties in MoS2/MoTe2 Hybrid Heterostructures for Dye-sensitized Solar Cells. Appl. Surf. Sci. 2020, 504, 144401. [Google Scholar] [CrossRef]
- Chia, W.S.; Kim, D.H.; Lee, C.S.; Park, J.T.; Kim, J.H. Highly Catalytic and Reflective Dual-phase Nickel Sulfide Electrodes for Solar Energy Conversion. Appl. Surf. Sci. 2018, 457, 1151–1157. [Google Scholar] [CrossRef]
- Zatirostami, A. A New Electrochemically Prepared Composite Counter Electrode for Dye-sensitized Solar Cells. Thin Solid Films 2020, 701, 137926. [Google Scholar] [CrossRef]
- Patni, N.; Pillai, S.G. Optimization of the Doping of Polyaniline via Response Surface Method to Prepare Polymer Electrolytes for Dye Sensitized Solar Cells. J. Environ. Chem. En. 2020, 8, 103709. [Google Scholar] [CrossRef]
- Jang, Y.J.; Thuy, C.T.T.; Thogiti, S.; Cheruku, R.; Ahn, K.S.; Kim, J.H. Electrochemically Deposited Polypyrrole for Counter Electrode of Quasi-solid-state Dye-sensitized Solar Cell. J. Nanosci. Nanotechnol. 2020, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kouhnavard, M.; Yifan, D.; Arcy, J.M.D.; Mishra, R.; Biswas, P. Highly Conductive PEDOT Films with Enhanced Catalytic Activity for Dye-sensitized Solar Cells. Sol. Energy 2020, 211, 258–264. [Google Scholar] [CrossRef]
- Cao, D.; Li, X.; Yu, X.; Cheng, N.; Yang, P.; Mi, B.; Gao, Z. Low-cost and Extra-simple Preparation of Porous NiS2 Counter Electrode for High-efficiency Dye-sensitized Solar Cells. Phys. Status Solidi A 2020, 217, 1900724. [Google Scholar] [CrossRef]
- Wang, M.; Anghel, A.M.; Marsan, B.; Ha, N.L.C.; Pootrakulchote, N.; Zakeeruddin, S.M.; Gratzel, M. CoS Supersedes Pt as Efficient Electrocatalyst for Triiodide Reduction in Dye-sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 15976–15977. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Lin, X.; Yu, N.; Wang, L.; Wang, L.; Hagfeldt, A.; Ma, T. Economical and Effective Sulfide Catalysts for Dye-sensitized Solar Cells as Counter Electrodes. Phys. Chem. Chem. Phys. 2011, 13, 19298–19301. [Google Scholar] [CrossRef]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Meng, Q. Dye-sensitized Solar Cells with NiS Counter Electrodes Electrodeposited by a Potential Reversal Technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Han, Q.; Hu, Z.; Wang, H.; Sun, Y.; Zhang, J.; Gao, L.; Wu, M. High Performance Metal Sulfide Counter Electrodes for Organic Sulfide Redox Couple in Dye-sensitized Solar Cells. Mater. Today Energy 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Sheela, S.E.; Murugadoss, V.; Sittaramane, R.; Angaiah, S. Development of Tungsten Diselenide/Polyaniline Composite Nanofibers as an Efficient Electrocatalytic Counter Electrode Material for Dye-sensitized Solar Cell. Sol. Energy 2020, 209, 538–546. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Li, C.T.; Huang, T.Y.; Lee, C.T.; Lin, C.Y.; Chu, C.W.; Vittal, R.; Ho, K.C. Electrocatalytic SiC Nanoparticles/PEDOT:PSS Composite Thin Films as the Counter Electrodes of Dye-sensitized Solar Cells. ChemElectroChem 2014, 1, 1031–1039. [Google Scholar] [CrossRef]
- Xu, T.; Cao, W.; Kong, D.; Qin, X.; Song, J.; Kou, K.; Chen, L.; Qiao, Q.; Huang, W. Enhanced Catalytic Property of Transparent PEDOT Counter Electrodes for Bifacial Dye Sensitized Solar Cells. Mater. Today Commun. 2020, 25, 101313. [Google Scholar] [CrossRef]
- Xu, T.; Kong, D.; Tang, H.; Qin, X.; Li, X.; Gurung, A.; Kou, K.; Chen, L.; Qiao, Q.; Huang, W. Transparent MoS2/PEDOT Composite Counter Electrodes for Bifacial Dye-sensitized Solar Cells. ACS Omega 2020, 5, 8687–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, T.; Ali, F.; Khalid, N.R.; Tahir, M.B.; Ijaz, M. Facile Synthesis and Antimicrobial Activity of CdS-Ag2S Nanocomposites. Bioorg. Chem. 2019, 90, 103064. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Su, Y.; Hu, Y.; Li, H.; Xie, W. Ag2S-CdS p-n Nanojunction-enhanced Photocatalytic Oxidation of Alcohols to Aldehydes. Small 2020, 16, 2001529. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhai, J.; Jiang, H.; Liu, D.; Zhang, L. CdS/Ag2S Nanocomposites Photocatalyst with Enhanced Visible Light Photocatalysis Activity. Solid State Sci. 2019, 98, 106020. [Google Scholar] [CrossRef]
- Di, T.; Cheng, B.; Ho, W.; Yu, J.; Tang, H. Hierarchically CdS–Ag2S Nanocomposites for Efficient Photocatalytic H2 Production. Appl. Surf. Sci. 2019, 470, 196–204. [Google Scholar] [CrossRef]
- Karimipour, M.; Shariat, M.; Sheida, R.; Molaei, M. A Visible to NIR Emission Conversion of CdS-Ag2S by Insertion of Graphene Oxide (GO) as Template Using Direct Atmospheric Plasma Injection. J. Lumin. 2019, 212, 334–341. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Zhang, Y.; Li, P. Ion-exchange Synthesis and Improved Photovoltaic Performance of CdS/Ag2S Heterostructures for Inorganic-organic Hybrid Solar Cells. Solid State Sci. 2016, 61, 195–200. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Sunlight-driven Competent Photocatalytic Degradation of Crystal Violet Using Sonochemically Produced GO Capped Ag2S Nanocomposites. Mater. Today Commun. 2019, 19, 157–169. [Google Scholar]
- Shao, M.; Wang, D.; Hu, B.; Yu, G.; Qian, Y. Self-template Route to CdS Hollow Spheres and in situ Conversion to CdS/Ag2S Composite Materials. J. Cryst. Growth 2003, 249, 549–552. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Jiang, W.; Zhang, M.; Ming, X.; Wang, S.; Zhang, Q. Strongly Visible-light Responsive Plasmonic Shaped AgX:Ag (X = Cl, Br) Nanoparticles for Reduction of CO2 to Methanol. Nanoscale 2012, 4, 5646–5650. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Zhang, J.; Cheng, B.; Yu, J. Enhanced Photocatalytic H2 Production on CdS Nanorod Using Cobalt-phosphate as Oxidation Cocatalyst. Appl. Surf. Sci. 2006, 389, 775–782. [Google Scholar] [CrossRef]
- Qiu, B.C.; Zhu, Q.H.; Du, M.M.; Fan, L.G.; Xing, M.Y.; Zhang, J.L. Efficient Solar Light Harvesting CdS/Co9S8 Hollow Cubes for Z-scheme Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Talooki, E.F.; Ghorbani, M.; Rahimnejad, M.; Lashkenari, M.S. Evaluation of a Visible Light-responsive Polyaniline Nanofiber-cadmium Sulfide Quantum Dots Photocathode for Simultaneous Hexavalent Chromium Reduction and Electricity Generation in Photo-microbial Fuel Cell. J. Electroanal. Chem. 2020, 873, 114469. [Google Scholar] [CrossRef]
- Hota, G.; Idage, S.B.; Khilar, K.C. Characterization of Nano-sized CdS-Ag2S Core-shell Nanoparticles Using XPS Technique. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 5–12. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Lee, R.H. Facile, Scalable, Eco-friendly Fabrication of High-performance Flexible All-solid-state Supercapacitors. Polymers 2018, 10, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyothibasu, J.P.; Kuo, D.A.; Lee, R.H. Flexible and Freestanding Electrodes Based on Polypyrrole/Carbon Nanotube/Cellulose Composites for Supercapacitor Application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Qin, Q.; Tao, J.; Yang, Y.; Dong, X. In situ Oxidative Polymerization of Polyaniline Counter Electrode on ITO Conductive Glass Substrate. Polym. Eng. Sci. 2011, 51, 663–669. [Google Scholar] [CrossRef]
- Ke, Z.Y.; Hung, H.C.; Lin, Y.J. Effects of Surface Modification of MoS2:TiO2:Pt Counter Electrodes by Argon Plasma Treatment on Photovoltaic Performance of Dye-sensitized Solar Cells. J Mater. Sci. Mater. Electron. 2017, 28, 4908–4913. [Google Scholar] [CrossRef]
- Raut, B.T.; Godse, P.R.; Pawar, S.G.; Chougule, M.A.; Bandgar, D.K.; Sen, S.; Patil, V.B. New Process for Fabrication of Polyaniline-CdS Nanocomposites: Structural Morphological and Optoelectronic Investigations. J. Phys. Chem. Solids 2013, 74, 236–244. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Luo, Y.; Guo, X.; Li, D.; Deng, M.; Huang, S.; Meng, Q. A Flexible Carbon Counter Electrode for Dye-sensitized Solar Cells. Carbon 2009, 47, 2704–2708. [Google Scholar] [CrossRef]
- Yeh, M.H.; Sun, C.L.; Su, J.S.; Lin, L.Y.; Lee, C.P.; Chen, C.Y.; Wu, C.G.; Vittal, R.; Ho, K.C. A Low-cost Counter Electrode of ITO Glass Coated with a Graphene/Nafion Composite Film for Use in Dye-sensitized Solar Cells. Carbon 2012, 50, 4192–4202. [Google Scholar] [CrossRef]














| DSSC | Counter Electrode | VOC (V) | JSC (mA cm−2) | FF | PCE (%) | Best PCE (%) |
|---|---|---|---|---|---|---|
| DSSC I | PANI-S | 0.71 ± 0.01 | 10.1 ± 0.02 | 0.54 ± 0.01 | 3.87 ± 0.14 | 4.01 |
| DSSC II | PACI-S | 0.71 ± 0.01 | 12.05 ± 0.01 | 0.57 ± 0.01 | 4.87 ± 0.17 | 5.04 |
| DSSC III | PACO-S | 0.64 ± 0.02 | 4.00 ± 0.02 | 0.27 ± 0.02 | 0.69 ± 0.08 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, M.; Cheng, T.-C.; Ye, H.-K.; Wang, T.-L.; Wu, T.-H.; Kuo, C.-C.; Lee, R.-H. Polyaniline/Ag2S–CdS Nanocomposites as Efficient Electrocatalysts for Triiodide Reduction in Dye-Sensitized Solar Cells. Catalysts 2021, 11, 507. https://doi.org/10.3390/catal11040507
Kuo M, Cheng T-C, Ye H-K, Wang T-L, Wu T-H, Kuo C-C, Lee R-H. Polyaniline/Ag2S–CdS Nanocomposites as Efficient Electrocatalysts for Triiodide Reduction in Dye-Sensitized Solar Cells. Catalysts. 2021; 11(4):507. https://doi.org/10.3390/catal11040507
Chicago/Turabian StyleKuo, Meng, Tsung-Chia Cheng, Huai-Kai Ye, Tzong-Liu Wang, Tzu-Ho Wu, Chi-Ching Kuo, and Rong-Ho Lee. 2021. "Polyaniline/Ag2S–CdS Nanocomposites as Efficient Electrocatalysts for Triiodide Reduction in Dye-Sensitized Solar Cells" Catalysts 11, no. 4: 507. https://doi.org/10.3390/catal11040507
APA StyleKuo, M., Cheng, T. -C., Ye, H. -K., Wang, T. -L., Wu, T. -H., Kuo, C. -C., & Lee, R. -H. (2021). Polyaniline/Ag2S–CdS Nanocomposites as Efficient Electrocatalysts for Triiodide Reduction in Dye-Sensitized Solar Cells. Catalysts, 11(4), 507. https://doi.org/10.3390/catal11040507