2.2.1. Equations
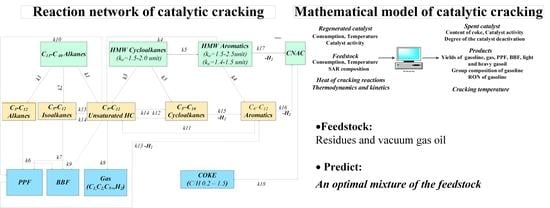
The formalized mechanism of cracking reactions simplifies the mathematical description of such a complicated multicomponent process. We developed a 14-lump kinetic model that includes hydrocarbon groups of the feedstock participating in the catalytic cracking. The reaction network is based on quantum–chemical methods and the database of hydrocarbons composition of the feedstock and products obtained at a laboratory of Tomsk Polytechnic University, Tomsk, Russia.
Table 3 contains the initial data to develop the model, including the concentration of the hydrocarbons groups of feedstock and products.
The predictive ability of the model depends on the set of desired products such as gasoline and fat gas. The developed reaction network (
Figure 2) helps predict the group composition of the gasoline, as well as the content of both the propane–propylene and butane–butylene fractions in wet gas, and coke. In addition, it also takes into account the SAR composition and the reactions leading to coke formation from these hydrocarbons on the catalyst. Therefore, this mechanism allows us to predict the amount of coke and the degree of catalyst deactivation when modeling the process of catalytic cracking.
The results of quantum–chemical methods of calculations confirmed the fundamental occurrence of reactions under the study (
Table 4). The probability of their occurrence according to isobaric–isothermal potential (ΔrG°
810–848), as well as the reactivity of hydrocarbon groups, were estimated at the equilibrium temperature between the feedstock and the catalyst (Equation (4)). Moreover, the thermal effects of reactions (ΔrH°
810–848) defined using DFT are used to solve a heat balance equation of the riser.
The mathematical model (Equation (1)) represents a system of ordinary differential equations describing the material and heat balances. The riser is modeled as a plug flow reactor, for the gas velocity (≈3.2–9.2 m/s) significantly exceeds the initial fluidizing velocity and the Peclet diffusion number tends to infinity.
Initial conditions: Ci = Ci,0, T0 = Ti.t.
Here, i is the number of components; j is the number of reactions; Ci is the concentration of i—the hydrocarbons group, mol/m3; τ is the contact time, s; j is the reaction number; ψ is the deactivation function (3); pm, cm are the density and heat capacity of flow, kg/m3, kJ/kg ∙ K; T is the temperature; , are the reaction rate in the forward and reverse directions, mol/(s·m3); Δr, Δr are the thermal effects of the chemical reactions, kJ/mol; Tit is the temperature of the thermal equilibrium between the feedstock and the catalyst, K;
A two-phase flow is required to describe the process with a moving catalyst bed. The passive phase is the gas–liquid flow, the active phase is the flow in pores of solid catalyst particles. The hydrodynamic mode of both phases is close to plug flow. The transfer of matter by moving solid particles of the catalyst is not important if the particles have a small specific surface area that poorly absorbs the reagent [
23].
The interdependence between reaction rates (18 reactions) and group concentrations (14 components) is based on the law of mass action according to the reaction network (
Figure 2). Moreover, the reaction rate is multiplied by the catalyst deactivation parameter when the concentration of the components calculates (Equation (2)):
The catalyst deactivation parameter
ψ is calculated depending on the content of coke on its surface. Using the results TPD, we defined the change in acid properties of the catalyst depending on the concentration of coke (Equation (3)):
where A is the current relative catalyst activity (acidity), %;
A0 is regenerated catalyst activity, %;
Ccoke is the coke content on the catalyst, wt%.
This approach allows us to implement the relationship among the following parameters: the composition of the feedstock—the content of coke on the catalyst—the change in catalyst activity—the change in the yield and composition of the products.
The model takes into account the interdependence between the operating variables of the riser and the regenerator. The cracking temperature depends largely on the temperature of the thermal equilibrium between the feedstock, the catalyst, and the heat of cracking reactions. The initial temperature of the reactions depends on the consumption of the feedstock and regenerated catalyst, as well as on their temperatures and heat capacities (Equation (4)):
where
Gcat is catalyst consumption, kg/s;
Gf is the feedstock consumption, kg/s;
cf,
ccat is the feedstock and the catalyst heat capacity, J/kgK;
Tcat is the temperature of regenerated catalyst,
K;
Tit is the temperature of the thermal equilibrium between the feedstock and the catalyst,
K.
Reaction rate constants were defined by solving the inverse kinetic problem, when the kinetic parameters are calculated based on the feedstock and product concentrations.
Table 3 contains initial data for solving the inverse kinetic problem. As an initial approximation, we used the kinetic parameters of the reaction of the individual hydrocarbons, which characterized the reaction groups, and lumps, which were defined both experimentally, including quantum-chemicals methods, and using the existent models [
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35].
Since the models differ significantly in reaction paths, the number of components, and their characteristics, as well as in catalysts type and operating variables etc., we used the kinetic parameters defined experimentally in several studies, where possible: for unsaturated hydrocarbons cyclization [
24], for C
5–C
12 alkanes and isoalkanes cracking [
25], for unsaturated hydrocarbons cracking [
26], for aromatics dealkylation [
27], for C
13–C
40 alkanes cracking [
28], for diene synthesis [
29], for hydrogen transfer [
30].
Our kinetic scheme is based on a group approach and includes several reactions when the feedstock converts into gasoline, and often, the products are both components of gas and gasoline. Although the use of the lump approach often involves the formation of a component through pseudo-reactions directly, without considering the specific hydrocarbons, we also take into account the modeling results: for C
5–C
12 isoalkanes cracking [
31], for condensation and coke formation [
32,
33], for cycloalkanes cracking and dealkylation [
34], for cycloalkanes cracking [
35].
At the same time, the most important factors are the catalyst nature and the operating variables, when the kinetic parameters are defined; most often, the databases present kinetic parameters of thermal conversion of individual hydrocarbons under a different catalyst.
These factors also lead to the optimization task of the kinetic parameters. To solve this problem, we used a genetic algorithm [
36,
37]. Finding the solution of the task using a genetic algorithm consists of the application of an iterative procedure, when the original solution improves gradually. At each iteration, several alternative candidate solutions (individuals) are considered immediately, and the genetic algorithm updates the population (the set of individuals used in the iteration) by creating new individuals and eliminating the worst ones.
The generation and mutation of new individuals is based on the modeling of the breeding and mutation processes using the crossing and the mutation operators. The reduction operator ensures the choice of the best (“revivable”) individuals among the parents and descendants by eliminating the worst (“poorly adapted”) ones. The latter performs according to strictly defined (deterministic) rules; at the same time, the crossing and the mutation occur randomly.
The main selection rule is the evolution law: “the strongest survives”, whichimprroves the finding solution. Equation (5) presents the expression for the fitness function:
where
yi is the value of the
i-th parameter (concentration);
yicalc is the calculated value of the
i-th parameter (concentration);
n is the total amount of parameters;
k is amount of days under the studied;
F is fitness function.
The implementation of these options provides a set of the kinetic parameters of the catalytic cracking reactions in accordance with reaction networks, which has a maximum fitness function. The degree of fitness of the kinetic parameters set is defined as 1/F.
Table 4 presents the estimated kinetic and thermodynamic parameters of the reactions involving high molecular weight hydrocarbons that convert into gas, gasoline, diesel fractions, and coke. Thermodynamic values marked with an asterisk were defined by quantum–chemical methods; for low molecular weight hydrocarbons, we used reference data [
38].
The defined set of kinetic parameters provides the sensitivity of the developed mathematical model to changes in the SAR composition of the feedstock, as well as in operating variables. In addition to the kinetic parameters, the thermodynamic parameters provide an accuracy of the model in terms of temperature and, consequently, the components concentrations. The mathematical model provides a quantitative account of the influence of the feedstock composition by considering the concentrations of reagents and reaction rate constants.
2.2.2. Verifications
The mathematical model was verified by comparing the calculated data of the product yields and industrial data (
Figure 3). A change in the main operating variables of the riser is presented in
Table 5. The feedstock composition changed according to
Table 6.
Verification of the model shows that the average relative error between the calculated and experimental data is not more than 7.0 wt%. (
Figure 3).
Figure 3d shows that the alkanes concentration with a step size h = 0.01 and h = 0.001 are close in comparison with h = 0.3–0.5, which ensures the convergence of the results during solving the system of ordinary differential equations.
Figure 4 shows the sensitivity of the model to SAR content in the feedstocks and the major process variable.
Model-based calculation shows that the gasoline yield is the highest (55.5–55.7 wt%) when the feedstocks with a high content of saturates are converted (C-1, C-2, and C-6 feed). At the same time, the values of RON of the gasoline are lower (90.48–92.15) relative to other feedstock, which have a high content of aromatics. Although the yields of gas are high values (24.6–24.9 wt%), the amount of light (10.81–11.12 wt%) gas oil is lower relative to other feeds.
The feedstock (F-10) which has a high concentration of aromatics (45.1 wt%) and resins (3.0 wt%), reduces the yield of the gasoline with a high octane number (95.69 units, 53.72 wt%) and gas (22.22 wt%). In addition, this feedstock contributes to the high rate of formation of coke (5.86 wt%), which leads to a decrease in the catalyst activity, as well as the degree of the feedstock conversion, and to an increase in heavy products: light and heavy gas oils (13.3 and 4.9 wt%).
Figure 5 and
Figure 6 show the effect of the main process variables on the cracking temperature and the latter on the consumption of product components.
Studying the parametric sensitivity showed that the change in the product yields and composition of cracking products is consistent with the theoretical laws about the process. With an increase in temperature, the consumption of feedstock components decreases and consumption of the gas components and the coke increases. The content of gasoline passes through a maximum due to an increase in the rates of cracking reactions with the release of gas components and condensation with further coke formation.
The developed model is intended to predict the yields and composition of products when the SAR composition of feedstock and the catalyst deactivation change. Such a model is to define the suitable ratio of the mixed feedstock for increasing the yield of the desirable product, taking into account the temperature, consumption of regenerated catalyst, as well as its deactivation.