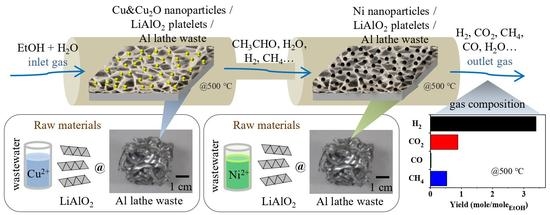
Synthesis of Catalytic Ni/Cu Nanoparticles from Simulated Wastewater on Li–Al Mixed Metal Oxides for a Two-Stage Catalytic Process in Ethanol Steam Reforming: Catalytic Performance and Coke Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characteristics
2.2. Effect of LiAlO2 Support and Cu/Ni Catalysts
2.3. Effect of Temperature on ESR over 6Ni Catalyst Configuration
2.4. Effect of Temperature on ESR over 6Cu_6Ni Catalyst Configuration
2.5. ESR vs. Reaction Time
2.6. Coke Properties
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Tests
3.3. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castelblanque, J.; Salimbeni, F. NF and RO membranes for the recovery and reuse of water and concentrated metallic salts from waste water produced in the electroplating process. Desalination 2004, 167, 65–73. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Uan, J.Y. Mg17Al12 phase in magnesium alloy waste facilitating the Ni2+ reduction in nickel plating wastewater. J. Hazard. Mater. 2021, 403, 123556. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Liao, C.H.; Huang, C.W.; Wu, J. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.H.; Uan, J.Y.; Hsu, T.L. Effects of concentrations of NaCl and organic acid on generation of hydrogen from magnesium metal scrap. Int. J. Hydrogen Energy 2012, 37, 3033–3040. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Abdul Wahid, M.; Jamil, M.; Azli, A.A.; Misbah, M.F. A review on biomass-based hydrogen production for renewable energy supply. Int. J. Energy Res. 2015, 39, 1597–1615. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen production by ethanol steam reforming (ESR) over CeO2 supported transition metal (Fe, Co, Ni, Cu) catalysts: Insight into the structure-activity relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sust. Energ. Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic reforming of oxygenates: State of the art and future prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freni, S.; Mondello, N.; Cavallaro, S.; Cacciola, G.; Parmon, V.; Sobyanin, V. Hydrogen production by steam reforming of ethanol: A two step process. React. Kinet. Catal. Lett. 2000, 71, 143–152. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M. A review of hydrotalcite based catalysts for hydrogen production systems. Int. J. Hydrogen Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Syu, J.H.; Uan, J.Y.; Lin, M.C.; Lin, Z.Y. Optically transparent Li–Al–CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Lin, M.C.; Chang, F.T.; Uan, J.Y. Aqueous Li+/Al3+ alkaline solution for CO2 capture and the massive Li–Al–CO3 hydrotalcite precipitation during the interaction between CO2 gas and the Li+/Al3+ aqueous solution. J. Mater. Chem. A. 2013, 1, 14773–14782. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Liu, S.J.; Uan, J.Y. Controllable luminescence of a Li–Al layered double hydroxide used as a sensor for reversible sensing of carbonate. J. Mater. Chem. C. 2019, 7, 11191–11206. [Google Scholar] [CrossRef]
- Stepanova, L.; Belskaya, O.; Vasilevich, A.; Gulyaeva, T.; Leont’eva, N.; Serkova, A.; Salanov, A.; Likholobov, V. The study of structural, textural and basic properties of MgAl-and LiAl-LDH prepared by mechanochemical method. Catal. Today 2019. [Google Scholar] [CrossRef]
- Di Cosimo, J.; Dıez, V.; Xu, M.; Iglesia, E.; Apesteguıa, C. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Castaldo, F.; Ricca, A.; Boettge, D. Ethanol steam reforming over bimetallic coated ceramic foams: Effect of reactor configuration and catalytic support. Int. J. Hydrogen Energy 2015, 40, 12650–12662. [Google Scholar] [CrossRef]
- Santander, J.A.; Tonetto, G.M.; Pedernera, M.N.; López, E. Ni/CeO2–MgO catalysts supported on stainless steel plates for ethanol steam reforming. Int. J. Hydrogen Energy 2017, 42, 9482–9492. [Google Scholar] [CrossRef]
- Palma, V.; Gallucci, F.; Pullumbi, P.; Ruocco, C.; Meloni, E.; Martino, M. Pt/Re/CeO2 Based Catalysts for CO-Water–Gas Shift Reaction: From Powders to Structured Catalyst. Catalysts 2020, 10, 564. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, C.H.; Yan, Y.Y.; Wang, M.K. Synthesis of Li/Al LDH using aluminum and LiOH. Appl. Clay Sci. 2013, 72, 191–195. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Gao, Y.; Qiao, Y.; Zheng, Q.; Guo, Z.; Zhao, Y.; O’Hare, D.; Wang, Q. Synthesis of LiAl2-layered double hydroxides for CO2 capture over a wide temperature range. J. Mater. Chem. A 2014, 2, 18454–18462. [Google Scholar] [CrossRef]
- Choong, C.K.; Huang, L.; Zhong, Z.; Lin, J.; Hong, L.; Chen, L. Effect of calcium addition on catalytic ethanol steam reforming of Ni/Al2O3: II. Acidity/basicity, water adsorption and catalytic activity. Appl. Catal. A 2011, 407, 155–162. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A 2012, 417, 281–289. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Steam reforming of acetic acid using Ni/Al2O3 catalyst: Influence of crystalline phase of Al2O3 support. Int. J. Hydrogen Energy 2017, 42, 20729–20738. [Google Scholar] [CrossRef]
- Chen, L.C.; Lin, S.D. The ethanol steam reforming over Cu-Ni/SiO2 catalysts: Effect of Cu/Ni ratio. Appl. Catal. B 2011, 106, 639–649. [Google Scholar] [CrossRef]
- Knapp, R.; Wyrzgol, S.A.; Jentys, A.; Lercher, J.A. Water–gas shift catalysts based on ionic liquid mediated supported Cu nanoparticles. J. Catal. 2010, 276, 280–291. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Peng, S.Y.; Zhang, M.J.; Lu, G.; Chen, Q.S.; Chen, Y.; Guo, G.C. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.-W.; Shi, C.; Ma, D. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.; Yoon, H.J.; Yoon, C.W.; Lee, K.B. Water gas shift and sorption-enhanced water gas shift reactions using hydrothermally synthesized novel Cu–Mg–Al hydrotalcite-based catalysts for hydrogen production. Renew. Sust. Energ. Rev. 2021, 145, 111064. [Google Scholar] [CrossRef]
- Marks, K.; Besharat, Z.; Soldemo, M.; Önsten, A.; Weissenrieder, J.; Stenlid, J.H.; Öström, H.; Göthelid, M. Adsorption and Decomposition of Ethanol on Cu2O (111) and (100). J. Phys. Chem. C 2019, 123, 20384–20392. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; de Freitas, I.C.; Longo, E.; Bueno, J.M.C. Site-selective ethanol conversion over supported copper catalysts. Catal. Commun. 2012, 26, 122–126. [Google Scholar] [CrossRef]
- Cunha, A.F.; Wu, Y.-J.; Li, P.; Yu, J.-G.; Rodrigues, A.E. Sorption-enhanced steam reforming of ethanol on a novel K–Ni–Cu–hydrotalcite hybrid material. Ind. Eng. Chem. Res. 2014, 53, 3842–3853. [Google Scholar] [CrossRef]
- Chen, F.; Tao, Y.; Ling, H.; Zhou, C.; Liu, Z.; Huang, J.; Yu, A. Ni-Cu bimetallic catalysts on Yttria-stabilized zirconia for hydrogen production from ethanol steam reforming. Fuel 2020, 280, 118612. [Google Scholar] [CrossRef]
- Dai, R.; Zheng, Z.; Lian, C.; Shi, K.; Wu, X.; An, X.; Xie, X. A sinter-resistant catalytic system based on ultra-small Ni–Cu nanoparticles encapsulated in Ca–SiO2 for high-performance ethanol steam reforming. Nanoscale 2020, 12, 16605–16616. [Google Scholar] [CrossRef]
- Niazi, Z.; Irankhah, A.; Wang, Y.; Arandiyan, H. Cu, Mg and Co effect on nickel-ceria supported catalysts for ethanol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 21512–21522. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, C.; Dai, R.; Wang, S.; Wu, X.; An, X.; Wu, Z.; Xie, X. Ethanol steam reforming on Ni-based catalysts: Effect of Cu and Fe addition on the catalytic activity and resistance to deactivation. Energy Fuels 2017, 31, 3091–3100. [Google Scholar] [CrossRef]
- Devrim, Y.; Albostan, A.; Devrim, H. Experimental investigation of CO tolerance in high temperature PEM fuel cells. Int. J. Hydrogen Energy 2018, 43, 18672–18681. [Google Scholar] [CrossRef]
- Ochoa, A.; Aramburu, B.; Ibáñez, M.; Valle, B.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Compositional insights and valorization pathways for carbonaceous material deposited during bio-oil thermal treatment. ChemSusChem 2014, 7, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- Montero, C.; Ochoa, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming in a fluidized bed. J. Catal. 2015, 331, 181–192. [Google Scholar] [CrossRef]
- Ochoa, A.; Arregi, A.; Amutio, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Coking and sintering progress of a Ni supported catalyst in the steam reforming of biomass pyrolysis volatiles. Appl. Catal. B 2018, 233, 289–300. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Velasquez, M.; Batiot-Dupeyrat, C.; Gallego, J.; Santamaria, A. Chemical and morphological characterization of multi-walled-carbon nanotubes synthesized by carbon deposition from an ethanol–glycerol blend. Diam. Relat. Mater. 2014, 50, 38–48. [Google Scholar] [CrossRef]
- Charisiou, N.; Tzounis, L.; Sebastian, V.; Hinder, S.; Baker, M.; Polychronopoulou, K.; Goula, M. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef] [Green Version]














| Site | CO2 Desorption | NH3 Desorption |
|---|---|---|
| Weak (%) | 7.32 | 5.29 |
| Medium (%) | 60.98 | 59.47 |
| Strong (%) | 31.71 | 35.24 |
| B/A a | 1.44 a | |
| Catalyst | XPS Compositions | |||
|---|---|---|---|---|
| O (at.%) | Al (at.%) | Ni (at.%) | Cu (at.%) | |
| Ni/LiAlO2 | 68.7 | 13.7 | 17.5 | -- |
| Cu/LiAlO2 | 71.8 | 16.0 | -- | 12.3 |
| Catalyst | Reaction Conditions | H2 Yield (%) | Gas Composition (vol.%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| S/E | Space Time (gcatal.·min/gEtOH) | H2 | CO2 | CO | CH4 | |||
| 6Ni a,b | 10 | 3.42 | 49.07 | 69.72 | 17.37 | 2.51 | 10.40 | This work |
| 6Cu_6Ni a,b | 10 | 3.42 | 56.63 | 70.32 | 18.32 | 0.48 | 10.88 | This work |
| Rh–Pd/CeO2 b,d | 6 | 10.04 | ~33 | ~53 | ~24 | ~3 | ~20 | [21] |
| Pt–Ni/CeO2–ZrO2 b,d | 3 | 6.39 | ~46 | ~60 | ~21 | ~6 | ~13 | [22] |
| Ni/CeO2–MgO b | 6 | 101.13 | ~53 | ~62 | ~23 | ~3 | ~12 | [23] |
| K-Cu–Ni/Mg–Al LDH c,d | 10 | 14.22 | ~67 | ~67 | ~21 | ~2 | ~10 | [39] |
| Cu–Ni/YSZ c,d | 3 | 8.15 | ~37 | ~61 | ~18 | ~8 | ~13 | [40] |
| Cu–Ni@Ca–SiO2 c,d | 6 | 8.33 | -- e | ~70 | ~15 | ~7 | ~8 | [41] |
| Cu–Ni/CeO2 c,d | 6 | 11.19 | -- e | ~69 | ~18 | ~2 | ~11 | [42] |
| Cu–Ni/Zeolite c,d | 8 | 8.16 | -- e | ~71 | ~20 | ~3 | ~6 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Huang, S.-H.; Uan, J.-Y.; Lin, H.-T. Synthesis of Catalytic Ni/Cu Nanoparticles from Simulated Wastewater on Li–Al Mixed Metal Oxides for a Two-Stage Catalytic Process in Ethanol Steam Reforming: Catalytic Performance and Coke Properties. Catalysts 2021, 11, 1124. https://doi.org/10.3390/catal11091124
Chen Y-J, Huang S-H, Uan J-Y, Lin H-T. Synthesis of Catalytic Ni/Cu Nanoparticles from Simulated Wastewater on Li–Al Mixed Metal Oxides for a Two-Stage Catalytic Process in Ethanol Steam Reforming: Catalytic Performance and Coke Properties. Catalysts. 2021; 11(9):1124. https://doi.org/10.3390/catal11091124
Chicago/Turabian StyleChen, Yu-Jia, Song-Hui Huang, Jun-Yen Uan, and Hao-Tung Lin. 2021. "Synthesis of Catalytic Ni/Cu Nanoparticles from Simulated Wastewater on Li–Al Mixed Metal Oxides for a Two-Stage Catalytic Process in Ethanol Steam Reforming: Catalytic Performance and Coke Properties" Catalysts 11, no. 9: 1124. https://doi.org/10.3390/catal11091124
APA StyleChen, Y. -J., Huang, S. -H., Uan, J. -Y., & Lin, H. -T. (2021). Synthesis of Catalytic Ni/Cu Nanoparticles from Simulated Wastewater on Li–Al Mixed Metal Oxides for a Two-Stage Catalytic Process in Ethanol Steam Reforming: Catalytic Performance and Coke Properties. Catalysts, 11(9), 1124. https://doi.org/10.3390/catal11091124