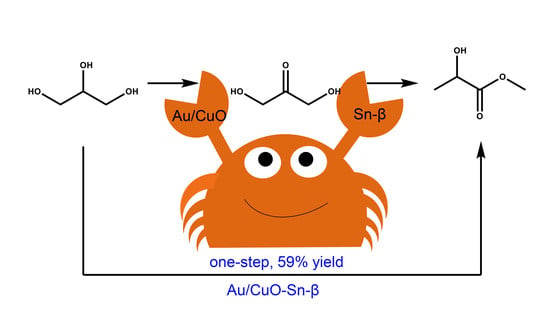
Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. XRD Patterns
2.1.2. FTIR Spectroscopy of Pyridine Adsorption
2.1.3. TEM Images
2.2. Effect of Sn-β on the Glycerol Oxidation
2.2.1. Sn Source and Sn Content
2.2.2. Preparation of Sn-β with Different Content of H2O in the Gel
2.3. Effect of Au/CuO on the GLY Oxidation
2.4. Effect of Reaction Condition
2.4.1. Temperature
2.4.2. Reaction Time
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.2.1. Preparation of Sn-β
3.2.2. Preparation of Au/CuO
3.2.3. Preparation of Au/CuO-Sn-β
3.3. Characterization
3.4. Oxidation of GLY
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2017, 118, 506–613. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Li, D.; Deng, D.; Ma, L.-F.; Yang, Y. Direct conversion of carbohydrates to diol by the combination of niobic acid and a hydrophobic ruthenium catalyst. RSC Adv. 2017, 7, 26487–26493. [Google Scholar] [CrossRef] [Green Version]
- Fulignati, S.; Antonetti, C.; Wilbers, E.; Licursi, D.; Heeres, H.J.; Raspolli Galletti, A.M. Tunable HMF hydrogenation to furan diols in a flow reactor using Ru/C as catalyst. J. Ind. Eng. Chem. 2021, 100, 390.e1. [Google Scholar] [CrossRef]
- Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. Effective hydrogenolysis of glycerol to 1,3-propanediol over metal-acid concerted Pt/WOx /Al2O3 Catalysts. ChemCatChem 2019, 11, 3903–3912. [Google Scholar] [CrossRef]
- Date, N.S.; Chikate, R.C.; Roh, H.-S.; Rode, C.V. Bifunctional role of Pd/MMT-K 10 catalyst in direct transformation of furfural to 1,2-pentanediol. Catal. Today 2018, 309, 195–201. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Trinh, Q.T.; Varghese, J.J.; Behling, R.; Valange, S.; Mushrif, S.H.; Jerome, F. Unraveling the mechanism of the oxidation of glycerol to dicarboxylic acids over a sonochemically synthesized copper oxide catalyst. Green Chem. 2018, 20, 2730–2741. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Guo, Y.; Liu, X.; Zhang, Y.; Wang, Y. N-doped carbon supported Pt catalyst for base-free oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Appl. Catal. A: Gen. 2016, 526, 1–8. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.B.; Liu, D.H. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.L.; Sun, Y.; Yang, S.L.; Tang, X.; Zeng, X.H.; Lin, L. An efficient approach to synthesizing 2,5-bis(N-methyl-aminomethyl)furan from 5-hydroxymethylfurfural via 2,5-bis(N-methyl-iminomethyl)furan using a two-step reaction in one pot. Green Chem. 2021, 23, 5656–5664. [Google Scholar] [CrossRef]
- Sha, J.; Kusema, B.T.; Zhou, W.J.; Yan, Z.; Streiff, S.; Pera-Titus, M. Single-reactor tandem oxidation-amination process for the synthesis of furan diamines from 5-hydroxymethylfurfural. Green Chem. 2021, 23, 7093–7099. [Google Scholar] [CrossRef]
- Xu, Y.M.; Jia, X.Q.; Ma, J.P.; Gao, J.; Xia, F.; Li, X.F.; Xu, J. Selective synthesis of 2,5-bis(aminomethyl) furan via enhancing the catalytic dehydrationhydrogenation of 2,5-diformylfuran dioxime. Green Chem. 2018, 20, 2697–2701. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Deng, D.; Li, D.; Zheng, M.; Duan, Y. Highly selective conversion of HMF to 1-hydroxy- 2,5-hexanedione on Pd/MIL-101(Cr). ChemistrySelect 2019, 4, 11165–11171. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, M.; Li, D.; Deng, D.; Ma, L.-F.; Yang, Y. Conversion of HMF to methyl cyclopentenolone by the Pd/Nb2O5 and Ca-Al catalysts via two-steps procedure. Green Chem. 2017, 19, 5103–5113. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Z.; Guo, W.; Liu, J.; Li, R.; Chen, R.; Huang, J. Hydrogenation and hydrolysis of furfural to furfuryl alcohol, cyclopentanone, and cyclopentanol with a heterogeneous copper catalyst in water. Ind. Eng. Chem. Res. 2019, 58, 3988–3993. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using Co-Ni catalyst in water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, D.; Zhang, C.; Zheng, M.; Duan, Y. Preparation of 1-hydroxy-2,5-hexanedione from HMF by the combination of commercial Pd/C and acetic acid. Molecules 2020, 25, 2475. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. Int. Edit. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Zhang, S.D.; Liu, Y.X.; Yang, S.; Li, H. Catalytic upgrading of bio-based 5-hydroxymethylfurfural to 2,5-dimethylfuran with non-noble metals. Energy Technol. 2021, 9, 2100653. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Miao, G.; Shi, L.; Zhou, Z.M.; Zhu, L.J.; Zhang, Y.F.; Zhao, X.P.; Luo, H.; Li, S.G.; Kong, L.Z.; Sun, Y.H. Catalyst design for selective hydrodeoxygenation of glycerol to 1,3-propanediol. ACS Catal. 2020, 10, 15217–15226. [Google Scholar] [CrossRef]
- Sun, S.Q.; Shu, L.; Lu, X.Y.; Wang, Q.H.; Tisma, M.; Zhu, C.G.; Shi, J.P.; Baganz, F.; Lye, G.J.; Hao, J. 1,2-Propanediol production from glycerol via an endogenous pathway of Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9003–9016. [Google Scholar] [CrossRef]
- Gabrysch, T.; Peng, B.X.; Bunea, S.; Dyker, G.; Muhler, M. The role of metallic copper in the selective hydrodeoxygenation of glycerol to 1,2-propanediol over Cu/ZrO2. ChemCatChem 2018, 10, 1344–1350. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Taher, H.; Banat, F. Development of Au and 1D hydroxyapatite nanohybrids supported on 2D boron nitride sheets as highly efficient catalysts for dehydrogenating glycerol to lactic acid. ACS Sustain. Chem. Eng. 2020, 8, 7278–7289. [Google Scholar] [CrossRef]
- Razali, N.; Abdullah, A.Z. Production of lactic acid from glycerol via chemical conversion using solid catalyst: A review. Appl. Catal. A Gen. 2017, 543, 234–246. [Google Scholar] [CrossRef]
- Torres, S.; Palacio, R.; Lopez, D. Support effect in Co3O4-based catalysts for selective partial oxidation of glycerol to lactic acid. Appl. Catal. A Gen. 2021, 621, 118199. [Google Scholar] [CrossRef]
- Qiu, K.B.; Shu, Y.X.; Zhang, J.; Gao, L.J.; Xiao, G.M. Effective and stable zeolite imidazole framework-supported copper nanoparticles (Cu/ZIF-8) for glycerol to lactic acid. Catal. Lett. 2021, 152, 172–186. [Google Scholar] [CrossRef]
- Palacio, R.; Torres, S.; Lopez, D.; Hernandez, D. Selective glycerol conversion to lactic acid on Co3O4/CeO2 catalysts. Catal. Today 2018, 302, 196–202. [Google Scholar] [CrossRef]
- Zavrazhnov, S.A.; Esipovich, A.L.; Zlobin, S.Y.; Belousov, A.S.; Vorotyntsev, A.V. Mechanism analysis and kinetic modelling of Cu NPs catalysed glycerol conversion into lactic acid. Catalysts 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Siddiki, S.; Touchy, A.S.; Kon, K.; Toyao, T.; Shimizu, K. Oxidant-free dehydrogenation of glycerol to lactic acid by heterogeneous platinum catalysts. ChemCatChem 2017, 9, 2816–2821. [Google Scholar] [CrossRef]
- Shen, Y.H.; Zhang, S.H.; Li, H.J.; Ren, Y.; Liu, H.C. Efficient synthesis of lactic acid by aerobic oxidation of glycerol on Au-Pt/TiO2 catalysts. Chem. A Eur. J. 2010, 16, 7368–7371. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, T.; Liu, X.; Ding, Y.J. Selective oxidation of glycerol to lactic acid over activated carbon supported Pt catalyst in alkaline solution. Chin. J. Catal. 2016, 37, 502–509. [Google Scholar] [CrossRef]
- Palacio, R.; Torres, S.; Royer, S.; Mamede, A.S.; Lopez, D.; Hernandez, D. CuO/CeO2 catalysts for glycerol selective conversion to lactic acid. Dalton Trans. 2018, 47, 4572–4582. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Jin, X.; Guan, Y.N.; Yin, B.; Chen, X.B.; Liu, Y.B.; Feng, X.; Shan, H.H.; Yang, C.H. Toward selective dehydrogenation of glycerol to lactic acid over bimetallic Pt-Co/CeOx catalysts. Ind. Eng. Chem. Res. 2019, 58, 14548–14558. [Google Scholar] [CrossRef]
- Dai, C.C.; Sun, L.B.; Liao, H.B.; Khezri, B.; Webster, R.D.; Fisher, A.C.; Xu, Z.C.J. Electrochemical production of lactic acid from glycerol oxidation catalyzed by AuPt nanoparticles. J. Catal. 2017, 356, 14–21. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Yaacob, M.H.; Basir, N.I. Synergy between oxides of Ni and Ca for selective catalytic lactic acid synthesis from glycerol in a single step process. J. Ind. Eng. Chem. 2020, 85, 282–288. [Google Scholar] [CrossRef]
- Yin, H.X.; Yin, H.B.; Wang, A.L.; Shen, L.Q. Catalytic conversion of glycerol to lactic acid over graphite-supported nickel nanoparticles and reaction kinetics. J. Ind. Eng. Chem. 2018, 57, 226–235. [Google Scholar] [CrossRef]
- Sharninghausen, L.S.; Campos, J.; Manas, M.G.; Crabtree, R.H. Efficient selective and atom economic catalytic conversion of glycerol to lactic acid. Nat. Commun. 2014, 5, 5084. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Fu, X.M.; Zhou, L.P.; Su, Y.L.; Yang, X.M.; Han, L.; Wang, J.F.; Song, C.Y. Promotion effect of Sn on Au/Sn-USY catalysts for one-pot conversion of glycerol to methyl lactate. ACS Catal. 2017, 7, 7274–7284. [Google Scholar] [CrossRef]
- Kwon, Y.; Birdja, Y.; Spanos, I.; Rodriguez, P.; Koper, M.T.M. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2012, 2, 759–764. [Google Scholar] [CrossRef]
- Xiao, Y.; Greeley, J.; Varma, A.; Zhao, Z.J.; Xiao, G.M. An experimental and theoretical study of glycerol oxidation to 1,3-dihydroxyacetone over bimetallic Pt-Bi catalysts. AlChE J. 2017, 63, 705–715. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in water. Chem. Sci. 2013, 4, 1820–1824. [Google Scholar] [CrossRef]
- An, Z.; Ma, H.H.; Han, H.B.; Huang, Z.Y.; Jiang, Y.T.; Wang, W.L.; Zhu, Y.R.; Song, H.Y.; Shu, X.; Xiang, X.; et al. Insights into the multiple synergies of supports in the selective oxidation of glycerol to dihydroxyacetone: Layered double hydroxide supported Au. ACS Catal. 2020, 10, 12437–12453. [Google Scholar] [CrossRef]
- Duan, X.Z.; Zhang, Y.F.; Pan, M.J.; Dong, H.; Chen, B.X.; Ma, Y.Y.; Qian, G.; Zhou, X.G.; Yang, J.; Chen, D. SbOx-promoted Pt nanoparticles supported on CNTs as catalysts for base-free oxidation of glycerol to dihydroxyacetone. AlChE J. 2018, 64, 3979–3987. [Google Scholar] [CrossRef]
- Walgode, P.M.; Faria, R.P.V.; Rodrigues, A.E. A review of aerobic glycerol oxidation processes using heterogeneous catalysts: A sustainable pathway for the production of dihydroxyacetone. Catal. Rev. 2021, 63, 422–511. [Google Scholar] [CrossRef]
- Hirasawa, S.; Nakagawa, Y.; Tomishige, K. Selective oxidation of glycerol to dihydroxyacetone over a Pd-Ag catalyst. Catal. Sci. Technol. 2012, 2, 1150–1152. [Google Scholar] [CrossRef]
- Hirasawa, S.; Watanabe, H.; Kizuka, T.; Nakagawa, Y.; Tomishige, K. Performance, structure and mechanism of Pd-Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2013, 300, 205–216. [Google Scholar] [CrossRef]
- Tan, H.; Yao, C.J.; Zhan, T.; Li, W.Q.; Zhu, J.P.; Wang, G.; Liu, W.B.; Sun, M.T.; Wang, S.H. Selective oxidation of glycerol to dihydroxyacetone over N-doped porous carbon stabilized CuxO supported Au catalysts. Mol. Catal. 2020, 498, 111243. [Google Scholar] [CrossRef]
- Ning, X.M.; Zhan, L.; Wang, H.J.; Yu, H.; Peng, F. Deactivation and regeneration of in situ formed bismuth-promoted platinum catalyst for the selective oxidation of glycerol to dihydroxyacetone. New J. Chem. 2018, 42, 18837–18843. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Hutchings, G.J. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002, 696–697. [Google Scholar] [CrossRef]
- Ke, Y.H.; Li, X.H.; Li, J.F.; Liu, C.L.; Xu, C.L.; Dong, W.S. Conversion of glycerol to dihydroxyacetone over Au catalysts on various supports. J. Chem. Technol. Biotechnol. 2020, 95, 1153–1162. [Google Scholar] [CrossRef]
- Innocenti, G.; Papadopoulos, E.; Fornasari, G.; Cavani, F.; Medford, A.J.; Sievers, C. Continuous liquid-phase upgrading of dihydroxyacetone to lactic acid over metal phosphate catalysts. ACS Catal. 2020, 10, 11936–11950. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mills, K.N.; Molley, A.M.; Rahaman, M.S.; Tulaphol, S.; Lalvani, S.B.; Dong, J.; Sunkara, M.K.; Sathitsuksanoh, N. Catalytic isomerization of dihydroxyacetone to lactic acid by heat treated zeolites. Appl. Catal. A Gen. 2021, 611, 117979. [Google Scholar] [CrossRef]
- Jolimaitre, E.; Delcroix, D.; Essayem, N.; Pinel, C.; Besson, M. Dihydroxyacetone conversion into lactic acid in an aqueous medium in the presence of metal salts: Influence of the ionic thermodynamic equilibrium on the reaction performance. Catal. Sci. Technol. 2018, 8, 1349–1356. [Google Scholar] [CrossRef]
- Sobus, N.; Czekaj, I. Comparison of synthetic and natural zeolite catalysts’ behavior in the production of lactic acid and ethyl lactate from biomass-derived dihydroxyacetone. Catalysts 2021, 11, 1006. [Google Scholar] [CrossRef]
- Wang, X.C.; Liang, F.B.; Huang, C.P.; Li, Y.X.; Chen, B.H. Siliceous tin phosphates as effective bifunctional catalysts for selective conversion of dihydroxyacetone to lactic acid. Catal. Sci. Technol. 2016, 6, 6551–6560. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Buszewski, B. Catalytic isomerization of dihydroxyacetone to lactic acid and alkyl lactates over hierarchical zeolites containing tin. Catalysts 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Pazhavelikkakath Purushothaman, R.K.; van Haveren, J.; Melian-Cabrera, I.; van Eck, E.R.; Heeres, H.J. Base-free, one-pot chemocatalytic conversion of glycerol to methyl lactate using supported gold catalysts. ChemSusChem 2014, 7, 1140–1147. [Google Scholar] [CrossRef]
- Palacio, R.; López, D.; Hernández, D. Bimetallic AuCu nanoparticles supported on CeO2 as selective catalysts for glycerol conversion to lactic acid in aqueous basic medium. J. Nanopart. Res. 2019, 21, 148. [Google Scholar] [CrossRef]
- Evans, C.D.; Douthwaite, M.; Carter, J.H.; Pattisson, S.; Kondrat, S.A.; Bethell, D.; Knight, D.W.; Taylor, S.H.; Hutchings, G.J. Enhancing the understanding of the glycerol to lactic acid reaction mechanism over AuPt/ TiO2 under alkaline conditions. J. Chem. Phys. 2020, 152, 134705. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fiorilli, S.L.; Heeres, H.J.; Pescarmona, P.P. Multifunctional heterogeneous catalysts for the selective conversion of glycerol into methyl lactate. ACS Sustain. Chem. Eng. 2018, 6, 10923–10933. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xu, Y.; Yang, X.; Lu, T.; Han, L. Utilization of biodiesel byproduct glycerol: Production of methyl lactate over Au/CuO and Sn-Beta binary catalyst under mild reaction conditions. Energy Convers. Manag. 2019, 196, 277–285. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Liu, C.L.; Xu, C.; Dong, W.S. The conversion of glycerol to methyl lactate catalyzed by tin-exchanged montmorillonite-supported gold catalysts. J. Chem. Technol. Biot. 2019, 94, 1958–1967. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Li, J.; Qi, X.; Guo, Z.; Wei, H.; Chu, H. Gold nanoparticles on nanosheets derived from layered rare-earth hydroxides for catalytic glycerol-to-lactic acid conversion. ACS Appl. Mater. Interfaces. 2021, 13, 522–530. [Google Scholar] [CrossRef]
- Mimura, N.; Muramatsu, N.; Hiyoshi, N.; Sato, O.; Yamaguchi, A. Continuous production of glyceric acid and lactic acid by catalytic oxidation of glycerol over an Au-Pt/Al2O3 bimetallic catalyst using a liquid-phase flow reactor. Catal. Today 2021, 375, 191–196. [Google Scholar] [CrossRef]
- Diguilio, E.; Renzini, M.S.; Pierella, L.B.; Domine, M.E. Conversion of glycerol to value added products in a semi-continuous batch reactor using noble metals supported on ZSM-11 zeolite. Nanomaterials 2021, 11, 510. [Google Scholar] [CrossRef] [PubMed]








| Entry | Catalyst | Conversion (%) | Yield (%) | |
|---|---|---|---|---|
| MLA | GLR | |||
| 1 | 1Au/2CuO-Sn-β | 6 | 3 | 1 |
| 2 | 1Au/3CuO-Sn-β | 25 | 6 | 2 |
| 3 | 1Au/4CuO-Sn-β | 89 | 56 | 16 |
| 4 | 1Au/6CuO-Sn-β | 82 | 54 | 17 |
| 5 | 1Au/9CuO-Sn-β | 67 | 39 | 12 |
| 6 | 1Au/12CuO-Sn-β | 32 | 16 | 5 |
| Entry | Temperature (K) | Conversion (%) | Yield (%) | |
|---|---|---|---|---|
| MLA | GLR | |||
| 1 | 343 | 53 | 27 | 7 |
| 2 | 363 | 89 | 56 | 16 |
| 3 | 393 | 98 | 54 | 11 |
| 4 | 413 | 97 | 32 | 6 |
| Entry | Catalyst | Product | T (K) | Conv. (%) | Sel. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1Au/4CuO-Sn-β | MLA | 363 | 88 | 67 | 59 | This work |
| 2 | Au/USY-600 | MLA | 433 | 95 | 77 | 73 | [63] |
| 3 | AuCu/CeO2 | lactic acid | 493 | 78 | 91 | 71 | [64] |
| 4 | AuPt/TiO2 | lactic acid | 373 | 100 | 68 | 68 | [65] |
| 5 | Au/CuO + Sn-MCM-41-XS | MLA | 413 | 95 | 66 | 63 | [66] |
| 6 | Au/CuO and Sn-Beta | MLA | 363 | 86 | 70 | 60 | [67] |
| 7 | Au/Sn(20)-Mt | MLA | 483 | 78 | 69 | 54 | [68] |
| 8 | Au/LPrO | lactic acid | 363 | 68 | 68 | 46 | [69] |
| 9 | Au-Pt | lactic acid | 358 | 90 | 44 | 40 | [70] |
| 10 | AuPt (15% Ptsurf) | lactic acid | r.t. | 30 | 73 | 22 | [40] |
| 11 | Au-Pt-ZSM-11 | lactic acid | 343 | 35 | 46 | 16 | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Luo, Q.; Nie, R.; Wang, J.; Zhang, Y.; Lu, T.; Xu, C. Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta. Catalysts 2022, 12, 104. https://doi.org/10.3390/catal12010104
Duan Y, Luo Q, Nie R, Wang J, Zhang Y, Lu T, Xu C. Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta. Catalysts. 2022; 12(1):104. https://doi.org/10.3390/catal12010104
Chicago/Turabian StyleDuan, Ying, Qianqian Luo, Renfeng Nie, Jianshe Wang, Yongsheng Zhang, Tianliang Lu, and Chunbao Xu. 2022. "Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta" Catalysts 12, no. 1: 104. https://doi.org/10.3390/catal12010104
APA StyleDuan, Y., Luo, Q., Nie, R., Wang, J., Zhang, Y., Lu, T., & Xu, C. (2022). Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta. Catalysts, 12(1), 104. https://doi.org/10.3390/catal12010104





_Xu.png)