A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Catalysts
2.2. Catalyst Characterization
2.2.1. BET and BJH Morphological Analyses
2.2.2. SEM and TEM Morphological Characterization
2.2.3. XRD Structural Characterization
2.2.4. FTIR Spectroscopy
2.2.5. TG-DTA Analysis
2.2.6. XPS Characterization
2.3. Catalytic Tests
3. Materials and Methods
3.1. Catalyst Synthesis
3.1.1. Materials
3.1.2. Functionalization of Silica by Aminopropyl Groups
3.1.3. Synthesis of Pd° NPs
3.1.4. Deposition of Pd° NPs on NH2-SiO2
3.1.5. Loading Pd-P Alloy on NH2-SiO2
3.2. Characterization Technics
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective Oxidation of Alcohols and Aldehydes over Supported Metal Nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Olenin, A.Y.; Mingalev, P.G.; Lisichkin, G.V. Partial Catalytic Oxidation of Alcohols: Catalysts Based on Metals and Metal Coordination Compounds (a Review). Pet. Chem. 2018, 58, 577–592. [Google Scholar] [CrossRef]
- Giang, L.T.K.; Ku, Y. Selective Oxidation of Benzyl Alcohol in Aqueous Phase by TiO2 Based Photocatalysts: A Review. Chem. Eng. Technol. 2021, 14, 2178–2190. [Google Scholar] [CrossRef]
- Wu, P.; Song, L.; Wang, Y.; Liu, X.; He, Z.; Bai, P.; Yan, Z. High-Performance Benzyl Alcohol Oxidation Catalyst: Au-Pd Alloy with ZrO2 as Promoter. Appl. Surf. Sci. 2021, 537, 148059. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Chaudhari, P.A.; Narkhede, V.S. Solvent-Free Liquid Phase Oxidation of Benzyl Alcohol to Benzaldehyde by Molecular Oxygen Using Non-Noble Transition Metal Containing Hydrotalcite-like Solid Catalysts. Catal. Commun. 2003, 4, 171–175. [Google Scholar] [CrossRef]
- Marotta, R.; Di Somma, I.; Spasiano, D.; Andreozzi, R.; Caprio, V. Selective Oxidation of Benzyl Alcohol to Benzaldehyde in Water by TiO2/Cu(II)/UV Solar System. Chem. Eng. J. 2011, 172, 243–249. [Google Scholar] [CrossRef]
- Ahmad, N.; Alam, M.; Adil, S.F.; Ansari, A.A.; Assal, M.E.; Ramay, S.M.; Ahmed, M.; Alam, M.M.; Siddiqui, M.R.H. Synthesis, Characterization, and Selective Benzyl Alcohol Aerobic Oxidation over Ni-Loaded BaFeO3 Mesoporous Catalyst. J. King Saud Univ. Sci. 2020, 32, 2059–2068. [Google Scholar] [CrossRef]
- Kunene, A.; Leteba, G.; van Steen, E. Liquid Phase Oxidation of Benzyl Alcohol over Pt and Pt–Ni Alloy Supported on TiO2: Using O2 or H2O2 as Oxidant? Catal. Lett. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Niu, S.; Shi, W.; Zhang, B.; Yu, W.; Xie, Y.; Ji, X.; Wu, Y.; Su, D.; Shao, L. Pd–P Nanoalloys Supported on a Porous Carbon Frame as an Efficient Catalyst for Benzyl Alcohol Oxidation. Catal. Sci. Technol. 2018, 8, 2333–2339. [Google Scholar] [CrossRef]
- Xin, P.; Li, J.; Xiong, Y.; Wu, X.; Dong, J.; Chen, W.; Wang, Y.; Gu, L.; Luo, J.; Rong, H.; et al. Revealing the Active Species for Aerobic Alcohol Oxidation by Using Uniform Supported Palladium Catalysts. Angew. Chem. 2018, 130, 4732–4736. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Chen, T.; Yang, Y. Surface-Functionalized TUD-1 Mesoporous Molecular Sieve Supported Palladium for Solvent-Free Aerobic Oxidation of Benzyl Alcohol. J. Catal. 2010, 275, 11–24. [Google Scholar] [CrossRef]
- Alshammari, H.M. Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Processes 2021, 9, 1590. [Google Scholar] [CrossRef]
- Yi, X.-T.; Zhao, T.; Wang, F.; Xu, J.; Xue, B. Palladium Nanoparticles Supported on Exfoliated G-C3N4 as Efficient Catalysts for Selective Oxidation of Benzyl Alcohol by Molecular Oxygen. New J. Chem. 2021, 45, 13519–13528. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Rossetti, I.; Villa, A.; Prati, L. Benzyl Alcohol Oxidation on Carbon-Supported Pd Nanoparticles: Elucidating the Reaction Mechanism. ChemCatChem 2014, 6, 3464–3473. [Google Scholar] [CrossRef]
- Sabaté, F.; Jordà, J.L.; Sabater, M.J. Ruthenium Isomorphic Substitution into Manganese Oxide Octahedral Molecular Sieve OMS-2: Comparative Physic-Chemical and Catalytic Studies of Ru versus Abundant Metal Cationic Dopants. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Mahdavi-Shakib, A.; Sempel, J.; Hoffman, M.; Oza, A.; Bennett, E.; Owen, J.S.; Rahmani Chokanlu, A.; Frederick, B.G.; Austin, R.N. Au/TiO2-Catalyzed Benzyl Alcohol Oxidation on Morphologically Precise Anatase Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 11793–11804. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective Benzyl Alcohol Oxidation over Pd Catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Plebani, M.; Schiavoni, M.; Milone, C.; Piperopoulos, E.; Galvagno, S.; Prati, L. Tuning Hydrophilic Properties of Carbon Nanotubes: A Challenge for Enhancing Selectivity in Pd Catalyzed Alcohol Oxidation. Catal. Today 2012, 186, 76–82. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Prati, L. Material Science for the Support Design: A Powerful Challenge for Catalysis. Catal. Sci. Technol. 2012, 2, 673–682. [Google Scholar] [CrossRef]
- Long, R.; Huang, H.; Li, Y.; Song, L.; Xiong, Y. Palladium-Based Nanomaterials: A Platform to Produce Reactive Oxygen Species for Catalyzing Oxidation Reactions. Adv. Mater. 2015, 27, 7025–7042. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Zhong, L.; Zhang, W. A Strategy to Immobilize Noble Metal Nanoparticles on Silica Microspheres. J. Mol. Catal. A Chem. 2010, 327, 92–100. [Google Scholar] [CrossRef]
- Fedorenko, S.; Jilkin, M.; Nastapova, N.; Yanilkin, V.; Bochkova, O.; Buriliov, V.; Nizameev, I.; Nasretdinova, G.; Kadirov, M.; Mustafina, A.; et al. Surface Decoration of Silica Nanoparticles by Pd(0) Deposition for Catalytic Application in Aqueous Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 486, 185–191. [Google Scholar] [CrossRef]
- Terra, J.C.S.; Moores, A.; Moura, F.C.C. Amine-Functionalized Mesoporous Silica as a Support for on-Demand Release of Copper in the A3-Coupling Reaction: Ultralow Concentration Catalysis and Confinement Effect. ACS Sustain. Chem. Eng. 2019, 7, 8696–8705. [Google Scholar] [CrossRef]
- Fihri, A.; Cha, D.; Bouhrara, M.; Almana, N.; Polshettiwar, V. Fibrous Nano-Silica (KCC-1)-Supported Palladium Catalyst: Suzuki Coupling Reactions Under Sustainable Conditions. ChemSusChem 2012, 5, 85–89. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M.; Zhiani, R.; Emrani, S. Pd/APTPOSS@KCC-1 as a New and Efficient Support Catalyst for C–H Activation. RSC Adv. 2017, 7, 24885–24894. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sabbaghi, A.; Huang, J.; Li, K.C.; Tsui, L.S.; Lam, F.L.Y.; Hu, X. Aerobic Oxidation of Benzyl Alcohol: Influence from Catalysts Basicity, Acidity, and Preparation Methods. Mol. Catal. 2020, 485, 110789. [Google Scholar] [CrossRef]
- Opanasenko, M.; Štěpnička, P.; Čejka, J. Heterogeneous Pd Catalysts Supported on Silica Matrices. RSC Adv. 2014, 4, 65137–65162. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lim, H.; Tang, Q.; Gao, Y.; Sun, T.; Yan, Q.; Yang, Y. Solvent-Free Aerobic Oxidation of Benzyl Alcohol over Pd Monometallic and Au–Pd Bimetallic Catalysts Supported on SBA-16 Mesoporous Molecular Sieves. Appl. Catal. A Gen. 2010, 380, 55–65. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, C.; Bin, D.; Wang, J.; Yan, B.; Shiraishi, Y.; Du, Y. Fabrication of Pd/P Nanoparticle Networks with High Activity for Methanol Oxidation. Catal. Sci. Technol. 2016, 6, 6441–6447. [Google Scholar] [CrossRef]
- Su, W.; Sun, R.; Ren, F.; Yao, Y.; Fei, Z.; Wang, H.; Liu, Z.; Xing, R.; Du, Y. Graphene Supported Palladium-Phosphorus Nanoparticles as a Promising Catalyst for Ethylene Glycol Oxidation. Appl. Surf. Sci. 2019, 491, 735–741. [Google Scholar] [CrossRef]
- Yang, Z.; Klabunde, K.J. Synthesis of Nearly Monodisperse Palladium (Pd) Nanoparticles by Using Oleylamine and Trioctylphosphine Mixed Ligands. J. Organomet. Chem. 2009, 694, 1016–1021. [Google Scholar] [CrossRef]
- Yoshida, R.; Sun, D.; Yamada, Y.; Sato, S. Stable Cu-Ni/SiO2 Catalysts Prepared by Using Citric Acid-Assisted Impregnation for Vapor-Phase Hydrogenation of Levulinic Acid. Mol. Catal. 2018, 454, 70–76. [Google Scholar] [CrossRef]
- Chen, Y. Chemical Preparation and Characterization of Metal–Metalloid Ultrafine Amorphous Alloy Particles. Catal. Today 1998, 44, 3–16. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Han, S.; Wang, X.; Wang, L.; Yu, W.; Wang, Z. Compositional Controls on Pore-Size Distribution by Nitrogen Adsorption Technique in the Lower Permian Shanxi Shales, Ordos Basin. J. Nat. Gas Sci. Eng. 2016, 34, 1369–1381. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.H.; Adam, F.; Muniandy, L. Green Synthesis of MCM-41 from Rice Husk and Its Functionalization with Nickel(II) Salen Complex for the Rapid Catalytic Oxidation of Benzyl Alcohol. Microporous Mesoporous Mater. 2020, 305, 110192. [Google Scholar] [CrossRef]
- Chandra, S.; Beaune, G.; Shirahata, N.; Winnik, F.M. A One-Pot Synthesis of Water Soluble Highly Fluorescent Silica Nanoparticles. J. Mater. Chem. B 2017, 5, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; McCue, A.J.; Miao, C.; Feng, J.; Li, D.; Anderson, J.A. Palladium Phosphide Nanoparticles as Highly Selective Catalysts for the Selective Hydrogenation of Acetylene. J. Catal. 2018, 364, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, M.; Feng, Z.; Qi, Y.; Feng, F.; Ma, L.; Zhang, Q.; Li, X. A Phosphorus–Carbon Framework over Activated Carbon Supported Palladium Nanoparticles for the Chemoselective Hydrogenation of Para-Chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar] [CrossRef]
- Belykh, L.B.; Skripov, N.I.; Sterenchuk, T.P.; Akimov, V.V.; Tauson, V.L.; Savanovich, T.A.; Schmidt, F.K. Role of Phosphorus in the Formation of Selective Palladium Catalysts for Hydrogenation of Alkylanthraquinones. Appl. Catal. A Gen. 2020, 589, 117293. [Google Scholar] [CrossRef]
- Wu, K.; Mao, X.; Liang, Y.; Chen, Y.; Tang, Y.; Zhou, Y.; Lin, J.; Ma, C.; Lu, T. Multiwalled Carbon Nanotubes Supported Palladium–Phosphorus Nanoparticles for Ethanol Electrooxidation in Alkaline Solution. J. Power Sources 2012, 219, 258–262. [Google Scholar] [CrossRef]
- Rego, R.; Ferraria, A.M.; Botelho do Rego, A.M.; Oliveira, M.C. Development of PdP Nano Electrocatalysts for Oxygen Reduction Reaction. Electrochim. Acta 2013, 87, 73–81. [Google Scholar] [CrossRef]
- Lv, H.; Sun, L.; Xu, D.; Ma, Y.; Liu, B. When Ternary PdCuP Alloys Meet Ultrathin Nanowires: Synergic Boosting of Catalytic Performance in Ethanol Electrooxidation. Appl. Catal. B Environ. 2019, 253, 271–277. [Google Scholar] [CrossRef]
- Okamoto, H. The P-Pd (Phosphorus-Palladium) System. JPE 1994, 15, 58–61. [Google Scholar] [CrossRef]
- Kucernak, A.R.J.; Fahy, K.F.; Sundaram, V.N.N. Facile Synthesis of Palladium Phosphide Electrocatalysts and Their Activity for the Hydrogen Oxidation, Hydrogen Evolutions, Oxygen Reduction and Formic Acid Oxidation Reactions. Catal. Today 2016, 262, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Q.; Wang, Y.; Wan, H. Size-Dependent Catalytic Activity of Supported Palladium Nanoparticles for Aerobic Oxidation of Alcohols. Adv. Synth. Catal. 2008, 350, 453–464. [Google Scholar] [CrossRef]
- Savara, A.; Rossetti, I.; Chan-Thaw, C.E.; Prati, L.; Villa, A. Microkinetic Modeling of Benzyl Alcohol Oxidation on Carbon-Supported Palladium Nanoparticles. ChemCatChem 2016, 8, 2482–2491. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Sutton, J.E.; Wang, D.; Prati, L.; Villa, A. Molecular Origin of the Selectivity Differences between Palladium and Gold–Palladium in Benzyl Alcohol Oxidation: Different Oxygen Adsorption Properties. ChemCatChem 2017, 9, 253–257. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Huang, X.; Yao, Y.; Willinger, M.; Shao, L.; Schlögl, R. Nanosized Palladium on Holey Graphene Sheets Incorporating PxOy for Effective Formic Acid Oxidation. Electrochem. Commun. 2017, 74, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Sahu, D.; Sarmah, C.; Das, P. A Highly Efficient and Recyclable Silica-Supported Palladium Catalyst for Alcohol Oxidation Reaction. Tetrahedron Lett. 2014, 55, 3422–3425. [Google Scholar] [CrossRef]
- Li, A.; Tang, S.; Tan, P.; Liu, C.; Liang, B. Measurement and Prediction of Oxygen Solubility in Toluene at Temperatures from 298.45 K to 393.15 K and Pressures up to 1.0 MPa. J. Chem. Eng. Data 2007, 52, 2339–2344. [Google Scholar] [CrossRef]
- Horstmann, S.; Grybat, A.; Kato, R. Experimental Determination and Prediction of Gas Solubility Data for Oxygen in Acetonitrile. J. Chem. Thermodyn. 2004, 36, 1015–1018. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Zhao, X.; Li, T.-J.; Chen, W.-L.; Kang, Q.-X.; Xu, X.-Q.; Liang, X.-X.; Feng, Y.; Duan, H.-H.; Lei, Z. Catalytic Properties of Palygorskite Supported Ru and Pd for Efficient Oxidation of Alcohols. Catal. Commun. 2015, 65, 34–40. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.J.; Gavriilidis, A. On the Development of Kinetic Models for Solvent-Free Benzyl Alcohol Oxidation over a Gold-Palladium Catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Particle Size and Dispersion Measurements. In Handbook of Heterogeneous Catalysis; American Cancer Society: Atlanta, GA, USA, 2008; pp. 738–765. ISBN 978-3-527-61004-4. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; ISBN 978-0-9627026-2-4. [Google Scholar]








| Technique | BET | BJH | BJH | SEM |
|---|---|---|---|---|
| Sample | S.A. | Pore Volume | Pore Size | Average Pd Particle Size |
| (m2 g−1) | (cm3 g−1) | (nm) | (nm) | |
| SiO2 | 564 | 0.63 | 4.3 | - |
| NH2-SiO2 | 88 | 0.17 | 10.8 | - |
| Pd°/NH2-SiO2 | 110 | 0.06 | 3.6 | 12 |
| Pd-P/NH2-SiO2 | 235 | 0.41 | 5.0 | 8 |
| Wave Number (cm−1) | Vibration |
|---|---|
| 3445–3420 | Stretching O-H |
| 2929, 2883 | Stretching CH2 |
| 1638 | Bending H2O |
| 1562 | Bending NH2 |
| 1495 | Bending CH2 |
| 1100–1045 | Asymmetric stretching Si-O-Si |
| 965 | Stretching Si-OH |
| 800 | Symmetric stretching Si-O-Si |
| 468 | Bending Si-O-Si |
| Samples | O 1s | C 1s | Pd 3d (5/2) | Pd 3d (3/2) | P 2p | N 1s | Si 2p |
|---|---|---|---|---|---|---|---|
| NH2-SiO2 | 532.96 (528.88) | 285.22 | - | - | - | 399.92 | 103.49 |
| Pd-P/NH2-SiO2 | 532.99 (530.15) | 285.00 | 335.28 337.55 1 | 340.37 342.22 1 | 130.39 | 399.77 | 103.57 |
| Sample | O 1s (%) | Pd 3d (%) | P 2p (%) | N 1s (%) | Si 2p (%) |
|---|---|---|---|---|---|
| NH2-SiO2 | 62.0 | - | - | 5.7 | 32.3 |
| Pd-P/NH2-SiO2 | 70.9 | 1.2 | 0.6 | 3.2 | 24.1 |
| Entry | Catalyst | Solvent | T (°C) | BnOH Conversion (%) 1 | PhCHO Selectivity (%) 1,2 |
|---|---|---|---|---|---|
| 1 | Pd°/NH2-SiO2 | Ethanol | 78 | <5 | N/A |
| 2 | Pd°/NH2-SiO2 | Toluene | 111 | <5 | N/A |
| 3 | Pd-P/NH2-SiO2 | Ethanol | 78 | 7 | 96 |
| 4 | Pd-P/NH2-SiO2 | Acetonitrile | 82 | 29 | 96 |
| 5 | Pd-P/NH2-SiO2 | Toluene | 80 | 38 | 94 |
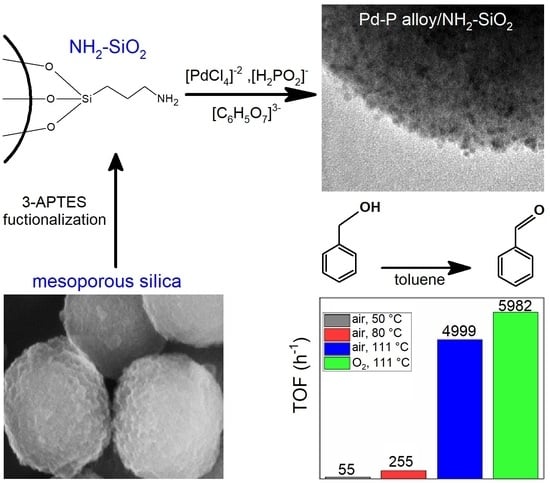
| Entry | T (°C) | Oxidant (20 mL min−1) | BnOH Conversion (%) 1 | PhCHO Selectivity (%) 1 | TOF (h−1) |
|---|---|---|---|---|---|
| 1 | 50 | Air | 11 | 65 | 55 |
| 2 | 80 | Air | 24 | 95 | 255 |
| 3 | 111 | Air | 57 | 63 | 4999 |
| 4 | 111 | O2 | 78 | 66 | 5982 |
| Catalyst | Support | Reaction Conditions | O2 (mL min−1) | T (°C) | TOF (h−1) | Reference |
|---|---|---|---|---|---|---|
| Pd-P/NH2-SiO2 | aminopropyl-functionalized mesoporous SiO2 | Solution | 20 | 111 | 5982 | This study |
| Pd°/NH2-SiO2 | <700 | |||||
| 1Pd/1.2APS-TUD | aminopropyl-functionalized TUD-1 silica | Solvent free | 20 | 100 | 2316 | [12] |
| 120 | 3251 | |||||
| Pd/APS-S16 | aminopropyl-functionalized SBA-16 silica | Solvent free | 20 | 100 | ~5600 | [29] |
| 110 | ~6070 | |||||
| Pd/PCF | porous carbon frame | Solvent free (continuous flow) | 5 | 70 | 2147 | [10] |
| Pd-P/PCF | 6289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeini, S.S.; Pasqual Laverdura, U.; Marconi, E.; Lisi, N.; Serra, E.; Chierchia, R.; Luisetto, I.; Tuti, S.; Tofani, D. A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 20. https://doi.org/10.3390/catal12010020
Moeini SS, Pasqual Laverdura U, Marconi E, Lisi N, Serra E, Chierchia R, Luisetto I, Tuti S, Tofani D. A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol. Catalysts. 2022; 12(1):20. https://doi.org/10.3390/catal12010020
Chicago/Turabian StyleMoeini, Seyed Sepehr, Umberto Pasqual Laverdura, Eleonora Marconi, Nicola Lisi, Emanuele Serra, Rosa Chierchia, Igor Luisetto, Simonetta Tuti, and Daniela Tofani. 2022. "A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol" Catalysts 12, no. 1: 20. https://doi.org/10.3390/catal12010020
APA StyleMoeini, S. S., Pasqual Laverdura, U., Marconi, E., Lisi, N., Serra, E., Chierchia, R., Luisetto, I., Tuti, S., & Tofani, D. (2022). A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol. Catalysts, 12(1), 20. https://doi.org/10.3390/catal12010020