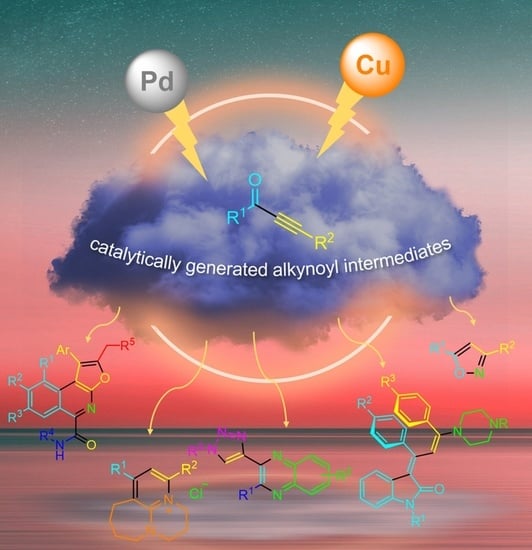
Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates
Abstract
:1. Introduction
2. Alkynone, Alkyl Propiolate, and Alkyne-1,2-Dione Formation by Catalytic Processes
3. Nitrogen Containing Heterocycles by Multicomponent Syntheses
3.1. Pyrazolines
3.2. Pyrazoles
3.3. Triazoles
3.4. Indolones
3.5. Pyridines
3.6. Pyrimidines
3.7. Isoquinolines
3.8. Quinoxalines
4. Oxygen Containing Heterocycles by Multicomponent Syntheses
4.1. Isoxazoles
4.2. Pyranones
4.3. Coumarins
4.4. Oxazaborinines
5. Thiophenes by Multicomponent Syntheses
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M. (Eds.) Multicomponent Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Müller, T.J.J. Relative Reactivities of Functional Groups as the Key to Multicomponent Reactions. In Multicomponent Reactions 1. General Discussion and Reactions Involving a Carbonyl Compound as Electrophilic Component; Müller, T.J.J., Ed.; Science of Synthesis Series; Georg Thieme Verlag KG: Stuttgart, Germany, 2014; pp. 5–27. [Google Scholar]
- Deilhof, K.; Müller, T.J.J. Alkynes in Multicomponent Synthesis of Heterocycles. In Multicomponent Reactions in Organic Synthesis; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 333–378. [Google Scholar]
- Müller, T.J.J. Synthesis of Heterocycles by Pd-catalyzed and Pd-catalysis Initiated Multi-Component Reactions. In Applied Homogeneous Catalysis with Organometallic Compounds; Beller, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 1463–1483. [Google Scholar]
- Algieri, V.; Algieri, C.; Maiuolo, L.; de Nino, A.; Pagliarani, A.; Tallarida, M.A.; Trombetti, F.; Nesci, S. 1,5-Disubstituted-1,2,3-triazoles as inhibitors of the mitochondrial Ca2+-activated F1Fo-ATP(hydrol)ase and the permeability transition pore. Ann. N. Y. Acad. Sci. 2021, 1485, 43–85. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Algieri, V.; Russo, B.; Tallarida, M.A.; Nardi, M.; di Gioia, M.L.; Merchant, Z.; Merino, P.; Delso, I.; de Nino, A. Synthesis, Biological and In Silico Evaluation of Pure Nucleobase-Containing Spiro (Indane-Isoxazolidine) Derivatives as Potential Inhibitors of MDM2-p53 Interaction. Molecules 2019, 24, 2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuolo, L.; Feriotto, G.; Algieri, V.; Nardi, M.; Russo, B.; di Gioia, M.L.; Furia, E.; Tallarida, M.A.; Mischiati, C.; de Nino, A. Antiproliferative Activity of Novel Isatinyl/Indanyl Nitrones (INs) as Potential Spin Trapping of Free Radical Intermediates. MedChemComm 2018, 9, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Müllen, K.; Wegner, G. (Eds.) Electronic Materials: The Oligomer Approach; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Walzer, K.; Maennig, B.; Pfeiffer, M.; Leo, K. Highly Efficient Organic Devices Based on Electrically Doped Transport Layers. Chem. Rev. 2007, 107, 1233–1271. [Google Scholar] [CrossRef] [PubMed]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. (Eds.) Organic Light Emitting Devices: Synthesis, Properties and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Sun, S.-S.; Dalton, L.R. Introduction to Organic Electronic and Optoelectronic Materials and Devices, 2nd ed.; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Mishra, A.; Fischer, M.K.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Levi, L.; Müller, T.J.J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 2016, 45, 2825–2846. [Google Scholar] [CrossRef] [Green Version]
- Levi, L.; Müller, T.J.J. Multicomponent Syntheses of Fluorophores Initiated by Metal Catalysis. Eur. J. Org. Chem. 2016, 2016, 2907–2918. [Google Scholar] [CrossRef]
- Müller, T.J.J. Multi-component Synthesis of Fluorophores via Catalytic Generation of Alkynoyl Intermediates. Drug Discov. Today Technol. 2018, 29, 19–26. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Müller, T.J.J. Multi-Component Syntheses of Heterocycles by Transition Metal Catalysis. Chem. Soc. Rev. 2007, 36, 1095–1108. [Google Scholar] [CrossRef]
- Müller, T.J.J. Palladium-Copper Catalyzed Alkyne Activation as an Entry to Multi-component Syntheses of Heterocycles. Top. Heterocycl. Chem. 2010, 25, 25–94. [Google Scholar]
- Whittaker, R.E.; Dermenci, A.; Dong, G. Synthesis of Ynones and Recent Application in Transition-Metal-Catalyzed Reactions. Synthesis 2015, 48, 161–183. [Google Scholar]
- Nájera, C.; Sydnes, L.K.; Yus, M. Conjugated Ynones in Organic Synthesis. Chem. Rev. 2019, 119, 11110–11244. [Google Scholar] [CrossRef]
- Gers-Panther, C.F.; Müller, T.J.J. Multicomponent Syntheses of Heterocycles Initiated by Catalytic Generation of Ynones and Ynediones. In Advances in Heterocyclic Chemistry: Heterocyclic Chemistry in the 21st Century: A Tribute to Alan Katritzky; Scriven, E.F.V., Ramsden, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 120, pp. 67–98. [Google Scholar]
- Willy, B.; Müller, T.J.J. Multi-component heterocycle syntheses via catalytic generation of alkynones. Curr. Org. Chem. 2009, 13, 1777–1790. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Tohda, Y.; Sonogashira, K.; Hagihara, N. A Convenient Synthesis of 1-Alkynyl Ketones and 2-Alkynamides. Synthesis 1977, 1977, 777–778. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Karpov, A.S.; Müller, T.J.J. New Entry to a Three-Component Pyrimidine Synthesis by TMS−Ynones via Sonogashira Coupling. Org. Lett. 2003, 5, 3451–3454. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Müller, T.J.J. Catalytic Alkynone Generation by Sonogashira Reaction and its Application in Three-component Pyrimidine Synthesis. Nat. Protoc. 2008, 3, 1660–1665. [Google Scholar] [CrossRef]
- Nordmann, J.; Breuer, N.; Müller, T.J.J. Efficient Consecutive Four-Component Synthesis of 5-Acylpyrid-2-ones Initiated by Copper-Free Alkynylation. Eur. J. Org. Chem. 2013, 2013, 4303–4310. [Google Scholar] [CrossRef]
- Boersch, C.; Merkul, E.; Müller, T.J.J. Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by In Situ Activation of Carboxylic Acids with Oxalyl Chloride. Angew. Chem. Int. Ed. 2011, 50, 10448–10452. [Google Scholar] [CrossRef]
- Merkul, E.; Oeser, T.; Müller, T.J.J. Consecutive Three-Component Synthesis of Ynones by Decarbonylative Sonogashira Coupling. Chem. Eur. J. 2009, 15, 5006–5011. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Müller, T.J.J. Rapid access to unsymmetrically substituted alkynes by sequentially palladium-catalyzed one-pot processes. Org. Biomol. Chem. 2016, 14, 3498–3500. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.M.; Mori, A. Carbonylative Sonogashira Coupling of Terminal Alkynes with Aqueous Ammonia. Org. Lett. 2003, 5, 3057–3060. [Google Scholar] [CrossRef]
- Karpov, A.S.; Merkul, E.; Rominger, F.; Müller, T.J.J. Concise Syntheses of Meridianins via Carbonylative Alkynylation and A Novel Four-Component Pyrimidine Synthesis. Angew. Chem. Int. Ed. 2005, 44, 6951–6956. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar-X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef]
- Cui, M.; Wu, H.; Jian, J.; Wang, H.; Liu, C.; Daniel, S.; Zeng, Z. Palladium-catalyzed Sonogashira coupling of amides: Access to ynones via C–N bond cleavage. Chem. Comm. 2016, 52, 12076–12079. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Michaelis, C.S.; Müller, T.J.J. 3-Phenothiazinyl propiolates—Fluorescent electrophores by Sonogashira coupling of ethyl propiolate. Dyes Pigm. 2017, 143, 308–316. [Google Scholar] [CrossRef]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L.A. Polyvinylpyridine-Supported Palladium Nanoparticles: A Valuable Catalyst for the Synthesis of Alkynyl Ketones via Acyl Sonogashira Reactions. Catal. Lett. 2020, 150, 652–659. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, M.; Gaur, R.; Gupta, R.; Adholeya, A.; Gawande, M.B. Synthesis of Iron Oxide Palladium Nanoparticles and Their Catalytic Applications for Direct Coupling of Acyl Chlorides with Alkynes. ChemPlusChem 2016, 81, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.K.; Ramachandra, S.N.; Shekharappa; Sureshbabu, V.V. Montmorillonite K-10-Supported Palladium Nanoparticles for Copper-Free Acyl Sonogashira Reaction. ChemistrySelect 2017, 2, 8059–8062. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, S.; Fang, Z.; Yang, Z.; Guo, K. Copper-catalyzed aerobic oxidative coupling of terminal alkynes with α-carbonyl aldehydes: An expedient approach toward ynediones. Tetrahedron Lett. 2019, 60, 150914. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Müller, T.J.J. Pyrazoles. In Science of Synthesis, Houben-Weyl; Carreira, E.M., Ed.; Science of Synthesis Knowledge Updates; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2017; Volume 3, pp. 1–128. [Google Scholar]
- Elguero, J.; Silva, A.M.S.; Tomé, A.C. Five-Membered Heterocycles: 1, 2-Azoles. Part 1. Pyrazoles. In Modern Heterocyclic Chemistry; Alvarez-Buila, J., Vaquero, J.J., Barluenga, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 2, pp. 635–725. [Google Scholar]
- Marella, A.; Rahmat, A.M.; Tauquir, A.M.; Saha, R.; Tanwar, O.; Akhter, M.; Shaquiquzzaman, M.; Mumtaz, A.M. Pyrazolines: A biological review. Mini. Rev. Med. Chem. 2013, 13, 921–931. [Google Scholar] [CrossRef]
- Görgen, C.; Boden, K.; Reiss, G.J.; Frank, W.; Müller, T.J.J. One-pot activation-alkynylation-cyclization synthesis of 1,5-diacyl-5-hydroxypyrazolines in a consecutive three-component fashion. Beilstein J. Org. Chem. 2019, 15, 1360–1370. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Theßeling, F.A.; Hoppe, C.; Müller, T.J.J. Rapid One-Pot Coupling-Coupling-Cyclocondensation Synthesis of Fluorescent Pyrazoles. J. Org. Chem. 2016, 81, 10328–10338. [Google Scholar] [CrossRef]
- Sakamoto, T.; Shiga, F.; Yasuhara, A.; Uchiyama, D.; Kondo, Y.; Yamanaka, H. Preparation of Ethyl Arylpropiolates from Aryl Iodides by Palladium-Catalyzed Cross-Coupling Reaction. Synthesis 1992, 1992, 746–748. [Google Scholar] [CrossRef]
- Niedballa, J.; Reiss, G.J.; Müller, T.J.J. Consecutive Three-Component Coupling-Addition Synthesis of β-Amino Enoates and 3-Hydroxypyrazoles via Ethyl 3-Arylpropiolates. Eur. J. Org. Chem. 2020, 2020, 5019–5024. [Google Scholar] [CrossRef]
- Niesobski, P.; Klukas, F.; Berens, H.; Makhloufi, G.; Janiak, C.; Müller, T.J.J. De novo Ring Forming Consecutive Four-component Syntheses of 4-Pyrazolyl-1,2,3-triazoles from TIPS-Butadiyne as a C4-Building Block. J. Org. Chem. 2018, 83, 4851–4858. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, F.; Yin, L.; Cai, M. A highly efficient heterogeneous palladium-catalyzed cascade three-component reaction of acid chlorides, terminal alkynes and hydrazines leading to pyrazoles. J. Organomet. Chem. 2016, 804, 108–113. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida., M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, X.; Jing, L.; Wu, G.; Kang, D.; Liu, X.; Zhan, P. Recent applications of click chemistry in drug discovery. Expert Opin. Drug Discov. 2019, 14, 779–789. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Lutz, J.F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I)-Catalyzed Huisgen Azide-Alkyne 1,3-Dipolar Cycloaddition Reaction in Nucleoside, Nucleotide, and Oligonucleotide Chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.-F. 1,3-Dipolar Cycloadditions of Azides and Alkynes: A Universal Ligation Tool in Polymer and Materials Science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC Reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Juríček, M.; Kouwer, P.H.J.; Rowan, A.E. Triazole: A unique building block for the construction of functional materials. Chem. Commun. 2011, 47, 8740–8749. [Google Scholar] [CrossRef] [Green Version]
- Espeel, P.; Du Prez, F.E. “Click”-Inspired Chemistry in Macromolecular Science: Matching Recent Progress and User Expectations. Macromolecules 2015, 48, 2–14. [Google Scholar] [CrossRef]
- Delaittre, G.; Guimard, N.K.; Barner-Kowollik, C. Cycloadditions in Modern Polymer Chemistry. Acc. Chem. Res. 2015, 48, 1296–1307. [Google Scholar] [CrossRef]
- Hayeebueraheng, A.; Kaewmee, B.; Rukachaisirikul, V.; Kaeobamrung, J. Synthesis of 2-(1,2,3-Triazolyl)benzamide Derivatives by a Copper(I)-Catalyzed Multicomponent Reaction. Eur. J. Org. Chem. 2017, 2017, 6714–6721. [Google Scholar] [CrossRef]
- Schreiner, E.; Wilcke, T.; Müller, T.J.J. A sequentially copper-catalyzed alkyne carboxylation-propargylation-azide cycloaddition (CuACPAC) synthesis of 1,2,3-triazolylmethyl arylpropiolates. Synlett 2016, 27, 379–382. [Google Scholar] [CrossRef]
- Nepveu, F.; Najahi, E.; Valentin, A. Antimalarial Activities of Indolones and Derivatives. Curr. Top. Med. Chem. 2014, 14, 1643–1652. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.Y.; Zhou, J. Therapeutic Potential of Spirooxindoles as Antiviral Agents. ACS Infect. Dis. 2016, 2, 382–392. [Google Scholar] [CrossRef]
- Schönhaber, J.; D’Souza, D.M.; Glißmann, T.; Mayer, B.; Janiak, C.; Rominger, F.; Frank, W.; Müller, T.J.J. Domino Insertion-Coupling Synthesis of Solid-State Luminescent Propynylidene Indolones. Chem. Eur. J. 2018, 24, 14712–14723. [Google Scholar] [CrossRef]
- Denißen, M.; Nirmalanathan, N.; Behnke, T.; Hoffmann, K.; Resch-Genger, U.; Müller, T.J.J. 3-Piperazinyl propenylidene indolone merocyanines—Consecutive three-component synthesis and electronic properties of solid state luminophores with AIE properties. Mater. Chem. Front. 2017, 1, 2013–2026. [Google Scholar] [CrossRef]
- Denißen, M.; Hannen, R.; Itskalov, D.; Biesen, L.; Nirmalananthan-Budau, N.; Hoffmann, K.; Reiss, G.J.; Resch-Genger, U.; Müller, T.J.J. One-pot Synthesis of a White-light Emissive Bichromophore Operated by Aggregation-induced Dual Emission (AIDE) and Partial Energy Transfer. Chem. Comm. 2020, 56, 7407–7410. [Google Scholar] [CrossRef] [PubMed]
- Elsner, A.-L.; Biesen, L.; Müller, T.J.J. Pseudo-Five-component Synthesis of Indolone-3-aminopropenylidene Merocyanine Dimers and Their Attenuated Aggregation-Induced Emission. Arkivoc 2021, 2021, 53–66. [Google Scholar] [CrossRef]
- McAteer, C.H.; Murugan, R.; Yamamoto, J.H. Pyridines and Their Benzo Derivatives: Applications. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Volume 7: Six-Membered Rings with One Heteroatom and Their Fused Carbocyclic Derivatives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 7.06, pp. 217–242. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. One-pot access to a privileged library of six membered nitrogenous heterocycles through multi-component cascade approach. Res. Chem. Intermed. 2016, 42, 5147–5196. [Google Scholar] [CrossRef]
- Driowya, M.; Saber, A.; Marzag, H.; Demange, L.; Benhida, R.; Bougrin, K. Microwave-Assisted Synthesis of Bioactive Six-Membered Heterocycles and Their Fused Analogues. Molecules 2016, 21, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohe, J.; Müller, T.J.J. Consecutive Three- and Four-component Coupling-Bagley-Bohlmann-Rahtz Syntheses of Tri- and Tetrasubstituted Pyridines. Z. Naturforsch. 2016, 71, 705–718. [Google Scholar] [CrossRef]
- Bakulina, O.; Merkt, F.K.; Knedel, T.O.; Janiak, C.; Müller, T.J.J. Water-soluble Blue Emissive Tricyclic 2-Aminopyridinium Salts by Three-component Coupling-(3+3)-Anellation Synthesis. Angew. Chem. Int. Ed. 2018, 57, 17240–17244. [Google Scholar] [CrossRef]
- Cao, T.; Martini, M.L.; Park, K.-S.; Kaniskan, H.Ü.; Jin, J. Pyrimidines and Their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Volume 8: Six-Membered Rings with One Heteroatom and Their Fused Carbocyclic Derivatives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 8.02, pp. 86–228. [Google Scholar] [CrossRef]
- Cheremnykh, K.P.; Savel’ev, V.; Shkurko, O.P.; Shults, E.E. Synthesis of hybrid molecules containing pyrimidine and diterpene alkaloid lappaconitine fragments. Chem. Heterocycl. Compd. 2018, 54, 1131–1138. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Moni, L.; Denißen, M.; Valentini, G.; Müller, T.J.J.; Riva, R. Diversity-oriented synthesis of intensively blue emissive 3-hydroxyisoquinolines by sequential Ugi four-component reaction-reductive Heck cyclization. Chem. Eur. J. 2015, 21, 753–762. [Google Scholar] [CrossRef]
- Moni, L.; Gers-Panther, C.F.; Anselmo, M.; Müller, T.J.J.; Riva, R. Highly Convergent Synthesis of Intensively Blue Emissive Furo[2,3-c]isoquinolines by a Palladium-catalyzed Cyclization Cascade of Unsaturated Ugi Products. Chem. Eur. J. 2016, 22, 2020–2031. [Google Scholar] [CrossRef]
- Huigens III, R.W.; Tenneti, S.; Xiao, T.; Garrison, A.T. Pyrazines and Their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Volume 8: Six-Membered Rings with One Heteroatom and Their Fused Carbocyclic Derivatives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 8.03, pp. 229–282. [Google Scholar] [CrossRef]
- Gers, C.F.; Nordmann, J.; Kumru, C.; Frank, W.; Müller, T.J.J. Highly Solvatochromic Fluorescent 2-Substituted 3-Ethynyl Quinoxalines—Four-component Synthesis, Photophysical Properties, and Electronic Structure. J. Org. Chem. 2014, 79, 3296–3310. [Google Scholar] [CrossRef]
- Gers-Panther, C.F.; Fischer, H.; Nordmann, J.; Seiler, T.; Behnke, T.; Würth, C.; Frank, W.; Resch-Genger, U.; Müller, T.J.J. Four- and Five-component Syntheses and Photophysical Properties of Emission Solvatochromic 3-Aminovinylquinoxalines. J. Org. Chem. 2017, 82, 567–578. [Google Scholar] [CrossRef]
- Merkt, F.K.; Höwedes, S.P.; Gers-Panther, C.F.; Gruber, I.; Janiak, C.; Müller, T.J.J. Three-component Activation−Alkynylation−Cyclocondensation (AACC) Synthesis of Enhanced Emission Solvatochromic 3-Ethynyl Quinoxalines. Chem. Eur. J. 2018, 24, 8114–8125. [Google Scholar] [CrossRef]
- Merkt, F.K.; Müller, T.J.J. Synthesis and Electronic Properties of Expanded 5-(Hetero)Aryl-Thien-2-yl Substituted 3-Ethynyl Quinoxalines with AIE Characteristics. Sci. China Chem. 2018, 61, 909–924. [Google Scholar] [CrossRef]
- Merkt, F.K.; Pieper, K.; Klopotowski, M.; Janiak, C.; Müller, T.J.J. Sequential Cu-catalyzed Four- and Five-Component Syntheses of Luminescent 3 Triazolylquinoxalines. Chem. Eur. J. 2019, 25, 9447–9455. [Google Scholar] [CrossRef]
- Cordero, F.M.; Giomi, D.; Machetti, F. Isoxazoles. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Volume 4: Five-Membered Rings with Two Heteroatoms and Their Fused Carbocyclic Derivatives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4.03, pp. 308–434. [Google Scholar]
- Görgen, C.; Müller, T.J.J. Facile Consecutive Three-Component Synthesis of 3,5-Disubstituted Isoxazoles. Chem. Heterocycl. Compd. 2017, 53, 422–429. [Google Scholar] [CrossRef]
- Thirukovela, N.S.; Balabiona, R.; Botla, V.; Vadde, R.; Jonnalagadda, S.B.; Vasam, C.S. One-pot regioselective synthesis of substituted pyrazoles and isoxazoles in PEG-400/water medium by Cu-free nano-Pd catalyzed sequential acyl Sonogashira coupling–intramolecular cyclization. Catal. Sci. Technol. 2019, 9, 6471–6481. [Google Scholar] [CrossRef]
- Deden, T.; May, L.; Reiss, G.J.; Müller, T.J.J. Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biarylsubstituted Isoxazoles. Catalysts 2020, 10, 1412. [Google Scholar] [CrossRef]
- Kaldre, D. Pyrans and Benzo Derivatives: Applications. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Volume 7: Six-Membered Rings with One Heteroatom and Their Fused Carbocyclic, Derivatives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 7.09, pp. 491–511. [Google Scholar] [CrossRef]
- Breuer, N.; Müller, T.J.J. Consecutive Alkynylation-Michael Addition-Cyclocondensation (AMAC) Multicomponent Syntheses of α-Pyrones and α-Pyridones. Synthesis 2018, 50, 2741–2752. [Google Scholar]
- Breuer, N.; Gruber, I.; Janiak, C.; Müller, T.J.J. Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1H-pyridines by Michael addition–cyclocondensation sequences. Beilstein J. Org. Chem. 2019, 15, 2684–2703. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, J.; Merkens, K.; Müller, T.J.J. Three-Component Synthesis and Photophysical Properties of Novel Coumarin-based Merocyanines. Chem. Eur. J. 2018, 24, 974–983. [Google Scholar] [CrossRef]
- Macedo, F.P.; Gwengo, C.; Lindeman, S.G.; Smith, M.D.; Gardinier, J.R. β-Diketonate, β-Ketoiminate, and β-Diiminate Complexes of Difluoroboron. Eur. J. Inorg. Chem. 2008, 2008, 3200–3211. [Google Scholar] [CrossRef]
- Dohe, J.; Koßmann, J.; Müller, T.J.J. Diversity-oriented Four-component Synthesis of Solid State Luminescent Difluoro Oxazaborinines. Dyes Pigm. 2018, 157, 198–217. [Google Scholar] [CrossRef]
- Bertaina-Anglade, V.; La Rochelle, C.D.; Scheller, D.K.A. Antidepressant properties of rotigotine in experimental models of depression. Eur. J. Pharmacol. 2006, 548, 106–114. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing pi-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Schaper, K.; Müller, T.J.J. Thiophene Syntheses by Ring Forming Multicomponent Reactions. Top. Curr. Chem. 2019, 376, 261–283. [Google Scholar]
- Teiber, M.; Giebeler, S.; Lessing, T.; Müller, T.J.J. Efficient pseudo-five-component coupling-Fiesselmann synthesis of luminescent oligothiophenes and their modification. Org. Biomol. Chem. 2013, 11, 3541–3552. [Google Scholar] [CrossRef]
- Breuer, N.; Müller, T.J.J. Synthesis and Electronic Properties of 5,5″-Diacceptor Substituted Terthiophenes. Dyes Pigm. 2018, 149, 676–685. [Google Scholar] [CrossRef]
































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedballa, J.; Müller, T.J.J. Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates. Catalysts 2022, 12, 90. https://doi.org/10.3390/catal12010090
Niedballa J, Müller TJJ. Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates. Catalysts. 2022; 12(1):90. https://doi.org/10.3390/catal12010090
Chicago/Turabian StyleNiedballa, Jonas, and Thomas J. J. Müller. 2022. "Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates" Catalysts 12, no. 1: 90. https://doi.org/10.3390/catal12010090
APA StyleNiedballa, J., & Müller, T. J. J. (2022). Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates. Catalysts, 12(1), 90. https://doi.org/10.3390/catal12010090