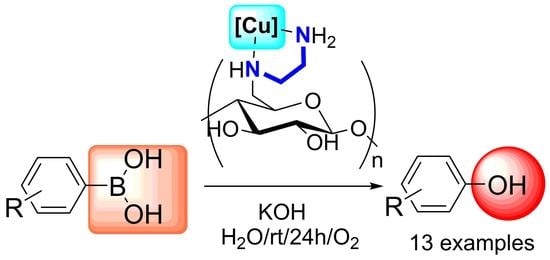
Application of Raw and Chemically Modified Biomasses for Heterogeneous Cu-Catalysed Conversion of Aryl boronic Acids to Phenols Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2. 13C Solid-State Nuclear Magnetic Resonance (13C SS NMR)
2.3. Ipso-Hydroxylation of Boronic Acids
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Cox and Box
3.2.2. Synthesis of CEDA
3.2.3. Synthesis of CEDA-[Cu]
3.2.4. Representative Procedure for Ipso-Hydroxylation of Boronic Acids
3.2.5. Preparation of Samples for Fourier Transform Infrared Spectroscopy (FTIR)
3.2.6. 13C Solid-State Nuclear Magnetic Resonance (13C SS NMR)
3.2.7. 1H Nuclear Magnetic Resonance (1H-NMR)
3.2.8. ICP Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, F.; Gurgel, L.V.A.; Soares, L.C.; Teodoro, F.S.; Ferreira, G.M.D.; Coelho, Y.L.; da Silva, L.H.M.; Prim, D.; Gil, L.F. Application of pyridine-modified chitosan derivative for simultaneous adsorption of Cu(II) and oxyanions of Cr(VI) from aqueous solution. J. Environ. Manag. 2021, 282, 111939. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Soares, L.C.; Teodoro, F.S.; Elias, M.M.C.; Savedra, R.M.L.; Siqueira, M.F.; Martineau-Corcos, C.; da Silva, L.H.M.; Prim, D.; Gurgel, L.V.A. Aminated cellulose as a versatile adsorbent for batch removal of As(V) and Cu(II) from mono- and multicomponent aqueous solutions. J. Colloid Interface Sci. 2020, 576, 158–175. [Google Scholar] [CrossRef]
- Gonçalves, F.J.; Kamal, F.; Gaucher, A.; Gil, R.; Bourdreux, F.; Martineau-Corcos, C.; Gurgel, L.V.A.; Gil, L.F.; Prim, D. Synthesis, characterization and application of pyridine-modified chitosan derivatives for the first non-racemic Cu-catalysed Henry reaction. Carbohydr. Polym. 2018, 181, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.R.; Rodrigues, J.A.V.; Adarme, O.F.H.; Melo, T.M.S.; Gurgel, L.V.A.; Gil, L.F. Optimization of cellulose and sugarcane bagasse oxidation: Application for adsorptive removal of crystal violet and auramine-O from aqueous solution. J. Colloid Interface Sci. 2017, 494, 223–241. [Google Scholar] [CrossRef]
- Joo, S.-R.; Kwon, G.-T.; Park, S.-Y.; Kin, S.-H. Chemically Modified Chitosan as a Biopolymer Support in Coppercatalyzed ipso-Hydroxylation of Arylboronic Acids in Water. Bull. Korean Chem. Soc. 2019, 40, 465–468. [Google Scholar] [CrossRef]
- Keshipour, S.; Shaabani, A. Copper(I) and palladium nanoparticles supported on ethylenediamine-functionalized cellulose as an efficient catalyst for the 1,3-dipolar cycloaddition/direct arylation sequence. Appl. Organomet. Chem. 2014, 28, 116–119. [Google Scholar] [CrossRef]
- Islam, T.; Rosales, J.A.; Saenz-Arana, R.; Ghadimi, S.J.; Noveron, J.C. Rapid synthesis of ultrasmall platinum nanoparticles supported on macroporous cellulose fibers for catalysis. Nanoscale Adv. 2019, 1, 2953–2964. [Google Scholar] [CrossRef] [Green Version]
- Prekob, Á.; Hajdu, V.; Muranszky, G.; Fiser, B.; Sycheva, A.; Ferenczi, T.; Viskolcz, B.; Vanyorek, L. Application of carbonized cellulose-based catalyst in nitrobenzene hydrogenation. Mater. Today Chem. 2020, 17, 100337. [Google Scholar] [CrossRef]
- Li, D.-D.; Lu, G.-P.; Cai, C. Modified cellulose with tunable surface hydrophilicity/hydrophobicity as a novel catalyst support for selective reduction of nitrobenzene. Catal. Commun. 2020, 137, 105949. [Google Scholar] [CrossRef]
- Santi, D.; Trisunaryanti, W.; Falah, I.I. Hydrocracking of pyrolyzed α-cellulose to hydrocarbon over MxOy/Mesoporous carbon catalyst (M = Co and Mo): Synthesis and characterization of carbon-based catalyst support from saw waste of Merbau wood. J. Environ. Chem. Eng. 2020, 8, 103735. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.; Tang, J.; Yan, N.; Du, Y.; Xi, S.; Wang, K.; Zhang, W.; Wen, H.; Wang, J. Immediate hydroxylation of arenes to phenols via V-containing all-silica ZSM-22 zeolite triggered non-radical mechanism. Nat. Commun. 2018, 9, 2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, N.; Shi, L.; Ma, Y.; Yang, X. Vanadium-zirconium catalyst on different support for hydroxylation of benzene to phenol with O2 as the oxidant. Appl. Catal. A 2018, 553, 117–125. [Google Scholar] [CrossRef]
- Tyman, J.H.P. Synthetic and Natural Phenols, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1996; Volume 52, pp. 1–700. [Google Scholar] [CrossRef]
- Rappoport, Z. The Chemistry of Phenols, 1st ed.; John Wiley and Sons: Oxford, UK, 2003; pp. 1–1667. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 589–621. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, P.; Zhang, X.; Wang, L. Trace amount Cu (ppm)-catalyzed intramolecular cyclization of 2-(gem-dibromovinyl)phenols(thiophenols) to 2-bromobenzofurans(thiophenes). Org. Biomol. Chem. 2013, 11, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhader, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Atia, A.A.; Kimura, M. Oxidative Hydroxylation of Aryl Boronic Acid Catalyzed by Co-porphyrin Complexes via Blue-Light Irradiation. Catalyst 2020, 10, 1262. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Wang, W.; Yang, Q.; Maa, Y.; Wang, K. Catalyst- and solvent-free ipso-hydroxylation of arylboronic acids to phenols. RSC Adv. 2019, 9, 34529. [Google Scholar] [CrossRef] [Green Version]
- Borah, R.; Saikia, E.; Bora, S.J.; Chetia, B. Banana pulp extract mediated synthesis of Cu2O nanoparticles: An efficient heterogeneous catalyst for the ipso-hydroxylation of arylboronic acids. Tetrahedron Lett. 2017, 58, 1211–1215. [Google Scholar] [CrossRef]
- Elumalai, V.; Hansen, J.H. A scalable and green one-minute synthesis of substituted phenols. RSC Adv. 2020, 10, 40582. [Google Scholar] [CrossRef]
- Molloy, J.J.; Clohessy, T.A.; Irving, C.; Anderson, N.A.; Lloyd-Jones, G.C.; Watson, A.J.B. Chemoselective oxidation of aryl organoboron systems enabled by boronic acid-selective phase transfer. Chem. Sci. 2017, 8, 1551. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, X.; Shao, C.; Su, D.; Cheng, G.; Hu, Y. Highly Efficient Synthesis of Phenols by Copper-Catalyzed Oxidative Hydroxylation of Arylboronic Acids at Room Temperature in Water. Org. Lett. 2010, 12, 1964–1967. [Google Scholar] [CrossRef]
- Kim, H.-S.; Joo, S.-R.; Shin, U.S.; Kim, S.-H. Recyclable CNT-chitosan nanohybrid film utilized in copper-catalyzed aerobic ipso-hydroxylation of arylboronic acids in aqueous media. Tetrahedron Lett. 2018, 59, 4597–4601. [Google Scholar] [CrossRef]
- Inamoto, K.; Nozawa, K.; Yonemoto, M.; Kondo, Y. Micellar system in copper-catalysed hydroxylation of arylboronic acids: Facile access to phenols. Chem. Commun. 2011, 47, 11775–11777. [Google Scholar] [CrossRef]
- Borah, R.; Saikia, E.; Bora, S.J.; Chetia, B. On-Water synthesis of phenols using biogenic Cu2O nanoparticles without using H2O2. RSC Adv. 2016, 6, 100443. [Google Scholar] [CrossRef]
- Dar, B.A.; Bhatti, P.; Singh, A.P.; Lazar, A.; Sharma, P.R.; Sharma, M.; Singh, B. Clay entrapped Cu(OH)x as an efficient heterogeneous catalyst for ipso-hydroxylation of arylboronic acids. Appl. Catal. A 2013, 466, 60–67. [Google Scholar] [CrossRef]
- Bora, S.J.; Chetia, B. Novel CuCl2-cryptand-[2.2.Benzo] complex: A base free and oxidant free catalyst for Ipso-Hydroxylation of aryl/heteroaryl-boronic acids in water at room temperature. J. Organomet. Chem. 2017, 851, 52–56. [Google Scholar] [CrossRef]
- Shin, E.-J.; Kim, H.-S.; Joo, S.-R.; Shin, U.S.; Kim, S.-H. Heterogeneous Palladium–Chitosan–CNT Core–Shell Nanohybrid Composite for Ipso-hydroxylation of Arylboronic Acids. Catalysis Lett. 2019, 149, 1560–1564. [Google Scholar] [CrossRef]
- Duan, P.; Schmidt-Rohr, K. Composite-pulse and partially dipolar dephased multiCP for improved quantitative solid-state 13C NMR. J. Magn. Reson. 2017, 285, 68–78. [Google Scholar] [CrossRef]
- Kono, H.; Yunoki, S.; Shikano, T.; Fujiwara, M.; Erata, T.; Takai, M. CP/MAS 13C NMR Study of Cellulose and Cellulose Derivatives. J. Am. Chem. Soc. 2002, 9, 7507. [Google Scholar] [CrossRef]
- Idström, A.; Schantz, S.; Sundberga, J.; Chmelka, B.F.; Gatenholm, P.; Nordstierna, L. 13C NMR assignments of regenerated cellulose from solid-state 2D NMR spectroscopy. Carbohydr. Polym. 2016, 151, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Dai, Y.; Wang, C.; Tan, L. Quantitative and Structure Analysis of Cellulose in Tobacco by 13C CP/MAS NMR Spectroscopy. Beiträge Tab. Int. 2016, 27, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Patil, B.H.; Peraje, P.; Naik, D.; Rajaramakrishna, R.; Dittmer, J.; Swamy, S.K.K. Experimental 1H and 13C Solid-State NMR Signal Assignment of Paramagnetic Copper (II) 2-Pyrazine-Carboxylate Complex using Density Functional Theory Calculations. J. Phys. Conf. Ser. 2021, 1819, 012032. [Google Scholar] [CrossRef]
- Yuan, C.; Zheng, L.; Zhao, Y. Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose. Molecules 2019, 24, 3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Li, Y.; Jiang, M.; Wang, J.; Fu, H. General Copper-Catalyzed Transformations of Functional Groups from Arylboronic Acids in Water. Chem. Eur. J. 2011, 17, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhu, H.; Driver, T.G. Cu-Catalyzed Cross-Coupling of Nitroarenes with Aryl Boronic Acids to Construct Diarylamines. ACS Catal. 2021, 11, 12417–12422. [Google Scholar] [CrossRef]
- Bian, Z.; Liu, A.; Li, Y.; Fang, G.; Yao, Q.; Zhange, G.; Wu, Z. Boronic acid sensors with double recognition sites: A review. Analyst 2020, 145, 719. [Google Scholar] [CrossRef] [PubMed]









| Entry | Solid Support | Conditions | Time (h) | Ratio 1/2 (a) |
|---|---|---|---|---|
| 1 | Cellulose | A | 24 | 1/0.8 |
| 2 | Bagasse | A | 24 | 1/0.5 |
| 3 | Cox | A | 24 | 1/0 |
| 4 | Box | A | 24 | 1/1.2 |
| 5 | CEDA | A | 24 | 0/1 |
| 6 | CEDA | B | 24 | 1/0 |
| 7 | CEDA | C | 24 | 1/0 |
| 8 | - | D | 24 | 1/0.5 |
| 9 | CEDA | A | 7 | 1/1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, F.G.; Teodoro, F.S.; Gurgel, L.V.A.; Bourdreux, F.; Zayene, O.; Gaucher, A.; Gil, L.F.; Prim, D. Application of Raw and Chemically Modified Biomasses for Heterogeneous Cu-Catalysed Conversion of Aryl boronic Acids to Phenols Derivatives. Catalysts 2022, 12, 92. https://doi.org/10.3390/catal12010092
Torres FG, Teodoro FS, Gurgel LVA, Bourdreux F, Zayene O, Gaucher A, Gil LF, Prim D. Application of Raw and Chemically Modified Biomasses for Heterogeneous Cu-Catalysed Conversion of Aryl boronic Acids to Phenols Derivatives. Catalysts. 2022; 12(1):92. https://doi.org/10.3390/catal12010092
Chicago/Turabian StyleTorres, Fernanda Guimarães, Filipe Simões Teodoro, Leandro Vinícius Alves Gurgel, Flavien Bourdreux, Olfa Zayene, Anne Gaucher, Laurent Frédéric Gil, and Damien Prim. 2022. "Application of Raw and Chemically Modified Biomasses for Heterogeneous Cu-Catalysed Conversion of Aryl boronic Acids to Phenols Derivatives" Catalysts 12, no. 1: 92. https://doi.org/10.3390/catal12010092
APA StyleTorres, F. G., Teodoro, F. S., Gurgel, L. V. A., Bourdreux, F., Zayene, O., Gaucher, A., Gil, L. F., & Prim, D. (2022). Application of Raw and Chemically Modified Biomasses for Heterogeneous Cu-Catalysed Conversion of Aryl boronic Acids to Phenols Derivatives. Catalysts, 12(1), 92. https://doi.org/10.3390/catal12010092