Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles
Abstract
:1. Introduction
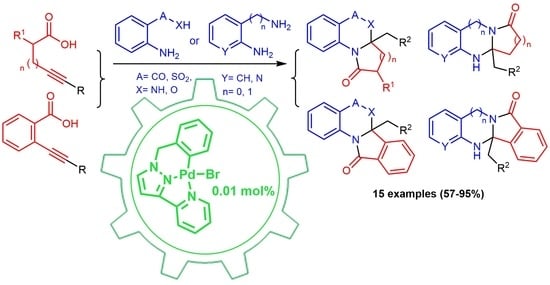
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Complex 1-Catalyzed Cascade Reaction between Alkynoic Acids and Heterodinucleophiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gopalaiah, K.; Choudhary, R. Synthesis of Kröhnke pyridines through iron-catalyzed oxidative condensation/double alkynylation/amination cascade strategy. Tetrahedron 2021, 98, 132429. [Google Scholar] [CrossRef]
- Liao, X.R.; Zhou, F.L.; Bin, Z.Y.; Yang, Y.D.; You, J.S. Palladium-Catalyzed Cascade Dearomative Spirocyclization and C-H Annulation of Aromatic Halides with Alkynes. Org. Lett. 2021, 23, 5203–5207. [Google Scholar] [CrossRef]
- Yang, X.H.; Huang, J.; Wang, F.; Liu, Z.L.; Li, Y.J.; Tao, C.A.; Wang, J.F. Copper-catalyzed alkynylation/annulation cascades of N-allyl ynamides: Regioselective access to medium-sized N-heterocycles. Org. Chem. Front. 2021, 8, 18–24. [Google Scholar] [CrossRef]
- Yang, Y.Z.; He, D.L.; Li, J.H. Rhodium-Catalyzed Reductive trans-Alkylacylation of Internal Alkynes via a Formal Carborhodation/C-H Carbonylation Cascade. Org. Lett. 2021, 23, 5039–5043. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Campbell, L.; Dixon, D.J. A Au(I)-Catalyzed N-Acyl Iminium Ion. Cyclization Cascade. J. Am. Chem. Soc. 2007, 129, 12070–12071. [Google Scholar] [CrossRef]
- Muratore, M.E.; Holloway, C.A.; Pilling, A.W.; Storer, R.I.; Trevitt, G.; Dixon, D.J. Enantioselective Brønsted Acid-Catalyzed N-Acyliminium Cyclization Cascades. J. Am. Chem. Soc. 2009, 131, 10796–10797. [Google Scholar] [CrossRef]
- Yang, T.; Ferrali, A.; Campbell, L.; Dixon, D.J. Combination iminium, enamine and copper(I) cascade catalysis: A carboannulation for the synthesis of cyclopentenes. Chem. Commun. 2008, 25, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Mutyala, A.K.; Lakshmi, P.G.V.; Gajula, B.; Sridhar, B.; Pottireddygari, G.R.; Rao, T.P. Au(I)-Catalyzed Cascade Reaction Involving Formal Double Hydroamination of Alkynes Bearing Tethered Carboxylic Groups: An Easy Access to Fused Dihydrobenzimidazoles and Tetrahydroquinazolines. J. Org. Chem. 2010, 75, 5963–5975. [Google Scholar] [CrossRef]
- Patil, N.T.; Shinde, V.S.; Sridhar, B. Relay Catalytic Branching Cascade: A Technique to Access Diverse Molecular Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 2251–2255. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-Catalyzed Tandem Transformation: A Simple Approach for the Synthesis of Pyrrolo/Pyrido[2,1-a][1,3]benzoxazinones and Pyrrolo/Pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Y.; Ji, X.; Liu, G.; Feng, E.; Ye, D.; Zhao, L.; Jiang, H.; Liu, H. Gold(I)-Catalyzed One-Pot Tandem Coupling/Cyclization: An Efficient Synthesis of Pyrrolo-/Pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv. Synth. Catal. 2010, 352, 373–378. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ji, X.; Zhou, W.; Zhang, X.; Qian, W.; Jiang, H.; Liu, H. Silver- and Gold-Mediated Domino Transformation: A Strategy for Synthesizing Benzo[e]indolo[1,2-a]pyrrolo/pyrido[2,1-c][1,4]diazepine-3,9-diones. J. Org. Chem. 2011, 76, 1239–1249. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhao, F.; Chen, X.; Zhang, L.; Jiang, H.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient syn-thesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Y.; Wang, L.; Zhao, L.; Jiang, H.; Liu, H. Au(I)/Ag(I)-catalyzed cascade approach for the synthesis of benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinones. J. Org. Chem. 2013, 78, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jia, X.; Li, P.; Liu, X.; Zhao, J.; Zhou, Y.; Wang, J.; Liu, H.; Zhao, F. Gold-catalyzed Rapid Construction of Nitrogen-containing Heter-ocyclic Compound Library with Scaffold Diversity and Molecular Complexity. Adv. Synth. Catal. 2019, 361, 1419–1440. [Google Scholar] [CrossRef]
- Jia, X.; Li, P.; Liu, X.; Lin, J.; Chu, Y.; Yu, J.; Wang, J.; Liu, H.; Zhao, F. Green and Facile Assembly of Diverse Fused N-Heterocycles Using Gold-Catalyzed Cascade Reactions in Water. Molecules 2019, 24, 988. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhai, Y.; Ji, X.; Liu, G.; Feng, E.; Ye, D.; Zhao, L.; Jiang, H.; Liu, H. Gold-Catalyzed One-Pot Cascade Construction of Highly Functionalized Pyrrolo[1,2-a]quinolin-1(2H)-ones. J. Org. Chem. 2009, 74, 7344–7348. [Google Scholar] [CrossRef]
- Naidu, S.; Reddy, S.R. Copper-catalyzed tandem reaction in ionic liquid: An efficient reusable catalyst and solvent media for the synthesis of fused poly hetero cyclic compounds. RSC Adv. 2016, 6, 62742–62746. [Google Scholar] [CrossRef]
- Naidu, S.; Reddy, S.R. A Green and Recyclable Copper and Ionic Liquid Catalytic System for the Construction of Polyheterocyclic Compounds via One-pot Tandem Coupling Reaction. ChemistrySelect 2017, 2, 1196–1201. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Lei, X. Ru-Catalyzed cascade reaction of α,ω-alkynoic acids and arylethylamines towards the synthesis of aryl-fused hetero-cycles. Org. Chem. Front. 2020, 7, 660–665. [Google Scholar] [CrossRef]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef] [PubMed]
- Brožič, P.; Turk, S.; Adeniji, A.O.; Konc, J.; Janežič, D.; Penning, T.M.; Rižner, T.L.; Gobec, S. Selective Inhibitors of Aldo-Keto Reductases AKR1C1 and AKR1C3 Discovered by Virtual Screening of a Fragment Library. J. Med. Chem. 2012, 55, 7417–7424. [Google Scholar] [CrossRef]
- Herrero, M.T.; Diaz de Sarralde, J.; SanMartin, R.; Bravo, L.; Dominguez, E. Cesium Carbonate-Promoted Hydroamidation of Alkynes: En-amides, Indoles and the Effect of Iron(III) Chloride. Adv. Synth. Catal. 2012, 354, 3054–3064. [Google Scholar] [CrossRef]
- Conde, N.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Palladium NNC Pincer Complex as an Efficient Catalyst for the Cycloisomerization of Alkynoic Acids. Adv. Synth. Catal. 2016, 358, 3283–3292. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.J.; Li, H.; Walsh, P.J. A Green Chemistry Approach to a More Efficient Asymmetric Catalyst: Solvent-Free and Highly Concentrated Alkyl Additions to Ketones. J. Am. Chem. Soc. 2005, 127, 16416–16425. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; El-Sayed, M.A. Enhancing Catalytic Efficiency of Hollow Palladium Nanoparticles by Photothermal Heating of Gold Nano-particles Added to the Cavity: Palladium–Gold Nanorattles. ChemCatChem 2014, 6, 3540–3546. [Google Scholar] [CrossRef]
- Nebra, N.; Monot, J.; Shaw, R.; Martin-Vaca, B.; Bourissou, D. 1,3-Bis(thiophosphinoyl)indene: A Unique and Versatile Scaffold for Original Polymetallic Complexes. ACS Catal. 2013, 3, 2930–2934. [Google Scholar] [CrossRef]
- Lambert, C.; Utimoto, K.; Nozaki, H. Palladium(II) catalyzed cyclization of alkynoic acids. Tetrahedron Lett. 1984, 25, 5323–5326. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ishii, Y.; Ishikawa, K.; Hidai, M. A Novel Catalyst with a Cuboidal PdMo3S4 Core for the Cyclization of Alkynoic Acids to Enol Lactones. Angew. Chem. Int. Ed. 1996, 35, 2123–2124. [Google Scholar] [CrossRef]
- Cordi, A.; Spedding, M.; Serkiz, B.; Lepagnol, J.; Desos, P.; Morain, P. Preparation of (3aS)-5,5-Dioxo-2,3,3a,4-tetrahydro-1H-pyrrolo[2,1-c][1,2,4]benzothiadiazine AMPA Receptor Agonist. Europen Patent EP692484, 17 January 1996. [Google Scholar]
- Gouliaev, A.H.; Larsen, M.; Varming, T.; Mathiesen, C.; Johansen, T.H.; Scheel-Krueger, J.; Olsen, G.M.; Nielsen, E.O. Preparation of Benzothiadiazines, Quinazolines, and Other Aryl-Fused Heterocycles as Positive AMPA-Receptor Modulators for Treatment of Memory and Learning Disorders. International Patent WO9942456, 26 August 1999. [Google Scholar]
- Francotte, P.; de Tullio, P.; Podona, T.; Diouf, O.; Fraikin, P.; Lestage, P.; Danober, L.; Thomas, J.-Y.; Caignard, D.-H.; Pirotte, P. Synthesis and pharmacological evaluation of a second generation of pyridothiadiazine 1,1-dioxides acting as AMPA potentiators. Bioorg. Med. Chem. 2008, 16, 9948–9956. [Google Scholar] [CrossRef]
- Carrozzo, M.M.; Battisti, U.M.; Cannazza, G.; Puia, G.; Ravazzini, F.; Falchicchio, A.; Perrone, S.; Citti, C.; Jozwiak, K.; Braghiroli, D.; et al. Design, stereoselective synthesis, configurational stability and biological activity of 7-chloro-9-(furan-3-yl)-2,3,3a,4-tetrahydro-1H-benzo[e]pyrrolo[2,1-c][1,2,4]thiadiazine 5,5-dioxide. Bioorg. Med. Chem. 2014, 22, 4667–4676. [Google Scholar] [CrossRef] [Green Version]
- Battisti, U.M.; Citti, C.; Rastelli, G.; Pinzi, L.; Puja, G.; Ravazzini, F.; Ciccarella, G.; Braghirolia, D.; Cannazza, G. An unexpected reversal in the pharmacological stereoselectivity of benzothiadiazine AMPA positive allosteric modulators. Med. Chem. Commun. 2016, 7, 2410–2417. [Google Scholar] [CrossRef]
- Destot-Wong, K.D.; Liang, K.; Gupta, S.K.; Favrais, G.; Schwendimann, L.; Pansiot, J.; Baud, O.; Spedding, M.; Lelièvre, V.; Mani, S.; et al. The AMPA receptor positive allosteric modulator, S18986, is neuroprotective against neonatal excitotoxic and inflammatory brain damage through BDNF synthesis. Neuropharmacology 2009, 57, 277–286. [Google Scholar] [CrossRef]
- Yefimenko, N.; Portero-Tresserra, M.; Marti-Nicolovius, M.; Guillazo-Blanch, G.; Vale-Martinez, A. The AMPA receptor modulator S18986 in the prelimbic cortex enhances acquisition and retention of an odor-reward association. Neurosci. Lett. 2013, 548, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaquin, S.; Jida, M.; Courtin, J.; Laconde, G.; Willand, N.; Deprez, B.; Deprez-Poulain, R. Water-based conditions for the microscale parallel synthesis of bicyclic lactams. Tetrahedron Lett. 2013, 54, 562–567. [Google Scholar] [CrossRef]
- De Sarro, A.; De Sarro, G.; Chimirri, A.; Grasso, S.; Monforte, A.-M.; Zappala, M. Anticonvulsant activity of pyrrolo[1’,2’:1,2]imidazo[4,5-b]pyridines, pyrrolo[2’,1’:2,3]imidazo[4,5-c]pyridines and pyrrolo[2,1-f]purines in DBA/2 mice. Gen. Pharmacol. 1994, 25, 1027–1031. [Google Scholar] [CrossRef]
- Caccamese, S.; Principato, G.; Chimirri, A.; Grasso, S. Separation of the enantiomers of anticonvulsant tricyclic pyrroloimidazolones by enanti-oselective HPLC. A chiral recognition model and a chiroptical study. Tetrahedron-Asymmetr. 1996, 7, 2577–2584. [Google Scholar] [CrossRef]
- Chimirri, A.; Grasso, S.; Monforte, P.; Zappalà, M.; Genchi, G. Pyrrolo[1’,2’:1,2]imidazo[4,5-b]pyridines, pyrrolo- [2’,1’:2,3]imidazo[4,5-c]pyridines and pyrrolo[2,1-f]purines as potential benzodiazepine ligands. Farmaco 1993, 48, 1261–1269. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Osburn, P.L.; Frels, J.D. Mechanistic Studies of SCS-Pd Complexes Used in Heck Catalysis. Adv. Synth. Catal. 2005, 347, 172–184. [Google Scholar] [CrossRef]
- Gorunova, O.N.; Novitskiy, I.M.; Grishin, Y.K.; Gloriozov, I.; Roznyatovsky, V.A.; Khrustalev, V.N.; Kochetkov, K.A.; Dunina, V.V. When Applying the Mercury Poisoning Test to Palladacycle-Catalyzed Reactions, One Should Not Consider the Common Misconception of Mercury(0) Selectivity. Organometallics 2018, 37, 2842–2858. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Sommer, W.; Richardson, J.M.; Weck, M.; Jones, C.W. Evidence that SCS Pincer Pd(II) Complexes are only Precatalysts in Heck Catalysis and the Implications for Catalyst Recovery and Reuse. Adv. Synth. Catal. 2005, 347, 161–171. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle hetero-geneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings–Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A. Distinguishing Between the Homogeneous and Heterogeneous Mechanisms of Catalysis in the Mizoroki-Heck and Suzuki-Miyaura Reactions: Problems and Prospects. Kinet. Catal. 2013, 53, 714–730. [Google Scholar] [CrossRef]
- CrysAlisPro, Agilent Technologies, Version 1.171.37.31 (Release 14 January 2014 CrysAlis171.NET) (Compiled 14 January 2014, 18:38:05). Available online: https://www.agilent.com/cs/library/usermanuals/Public/CrysAlis_Pro_User_Manual.pdf (accessed on 10 January 2022).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–342. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0–New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Aeberli, P.; Houlihan, W.J. Reaction of some oxo acids with anthranilic acid, anthranilamides, orthanilamides, and salicylamide. J. Org. Chem. 1968, 33, 2402–2407. [Google Scholar] [CrossRef]
- Hadfield, J.A.; Pavlidis, V.H.; Roffey, J.R.A. Synthesis of Cytotoxic Pyrrolo[1,2-a]-benzimidazol-1-ones. Synth. Commun. 1995, 25, 1319–1329. [Google Scholar] [CrossRef]










| Entry | Poisoning Additive | 4a (%) b |
|---|---|---|
| 1 | Hg (one drop) | 99 |
| 2 | CS2 (0.5 equiv. per metal atom) | 99 |
| 3 | CS2 (2.0 equiv. per metal atom) | 98 |
| 4 | PPh3 (0.03 equiv. per metal atom) | 99 |
| 5 | PPh3 (0.3 equiv. per metal atom) | 99 |
| 6 | PPh3 (4.0 equiv. per metal atom) | 99 |
| 7 | Py (150 equiv. per metal atom) c | 98 |
| 8 | PVPy (300 equiv. per metal atom) d | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, N.; Herrero, M.T.; Urgoitia, G.; SanMartin, R. Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles. Catalysts 2022, 12, 127. https://doi.org/10.3390/catal12020127
Conde N, Herrero MT, Urgoitia G, SanMartin R. Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles. Catalysts. 2022; 12(2):127. https://doi.org/10.3390/catal12020127
Chicago/Turabian StyleConde, Nerea, María Teresa Herrero, Garazi Urgoitia, and Raul SanMartin. 2022. "Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles" Catalysts 12, no. 2: 127. https://doi.org/10.3390/catal12020127
APA StyleConde, N., Herrero, M. T., Urgoitia, G., & SanMartin, R. (2022). Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles. Catalysts, 12(2), 127. https://doi.org/10.3390/catal12020127