Full and Sustainable Electrochemical Production of Chlorine Dioxide
Abstract
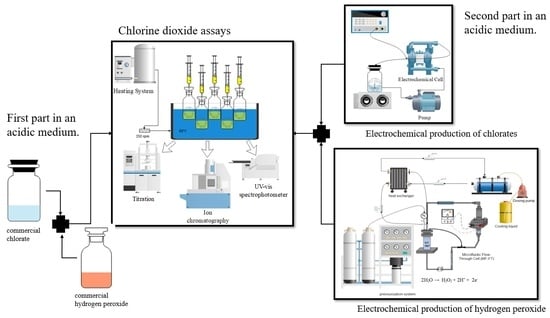
:1. Introduction
2. Results and Discussion
2.1. Chemical Production of Chlorine Dioxide
2.2. Electrochemical Production of Chlorate and Hydrogen Peroxide
2.3. Production of Chlorine Dioxide by Combination of Electrolytic Solutions
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Setup
3.2.1. Electrochemical Cells
3.2.2. Chlorine Dioxide Assays
3.3. Characterization Methods
4. Conclusions
- Hydrogen peroxide and chlorate can be efficiently produced by the electrolysis of perchlorate solutions and sodium chloride solutions, respectively. Applying 5.0 mA cm−2, 2 bars of a solution containing 118.8 mg L−1 H2O2 can be obtained after passing 0.71 Ah L−1 (8 h of electrolysis). By applying 50.0 mA cm−2, a solution containing 10,100.1 mg L−1 of chlorate can be obtained after passing 48.44 Ah L−1 (4 h of electrolysis). The first solution contains 3000 mg L−1 of perchloric acid while the second 20 g L−1 of sodium chloride.
- The reaction between commercial chlorate and hydrogen peroxide solutions led to the formation of chlorine dioxide. The reaction is highly reproducible, and stoichiometry is very important, because chlorine dioxide is not the final product but an intermediate in the reduction of chlorates to chlorine by hydrogen peroxide. In addition to suitable ratios of H2O2/ClO3−, it is important that in the reaction mixture chlorate is not exhausted, in order to prevent further reduction of ClO2
- Mixing of the two electrochemically produced reagents with sulfuric acid leads to the successful formation of chlorine dioxide. Maximum conversions close to 90% of the chlorate dose are obtained. Using the electrochemically-produced solutions, the significance of selecting a suitable ratio between reagents and the necessity of surplus chlorate in solution to prevent further reduction of chlorine dioxide is also confirmed.
- A gas stream with a high oxidation capacity and consisting mainly of chloride dioxide is produced either when mixing chemical or electrochemically produced solutions of hydrogen peroxide and chlorates. This confirms the good prospects of the technology for the manufacturing of portable electrochemically-based chlorine dioxide production units
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jonnalagadda, S.B.; Nadupalli, S. Chlorine Dioxide for Bleaching, Industrial Applications and Water Treatment. Indian Chem. Eng. 2014, 56, 123–136. [Google Scholar] [CrossRef]
- Luo, J.; Christensen, P.K. Sodium-Chlorite as an Alternative for Chlorine Dioxide in Pulp Bleaching. Appita J. 1992, 45, 38–40. [Google Scholar]
- Bergmann, H.; Koparal, A.S. The formation of chlorine dioxide in the electrochemical treatment of drinking water for disinfection. Electrochim. Acta 2005, 50, 5218–5228. [Google Scholar] [CrossRef]
- Wang, L.-S.; Zhang, T.; Hu, H.-Y. Comparison of the quality and toxicity of wastewater after chlorine and chlorine dioxide disinfections. Huan Jing Ke Xue 2005, 26, 75–78. [Google Scholar] [PubMed]
- Schmidt, W. Using chlorine dioxide for drinking water a disinfection by the application of the chlorite/chlorine process. Acta Hydrochim. Hydrobiol. 2004, 32, 48–60. [Google Scholar] [CrossRef]
- Benarde, M.A.; Snow, W.B.; Olivieri, V.P.; Davidson, B. Kinetics and mechanism of bacterial disinfection by chlorine dioxide. Appl. Microbiol. 1967, 15, 257–265. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Biasibetti, M.; Collivignarelli, C. Influence of drinking water treatments on chlorine dioxide consumption and chlorite/chlorate formation. Water Res. 2014, 54, 44–52. [Google Scholar] [CrossRef]
- Hubbard, H.; Poppendieck, D.; Corsi, R.L. Chlorine Dioxide Reactions with Indoor Materials during Building Disinfection: Surface Uptake. Environ. Sci. Technol. 2009, 43, 1329–1335. [Google Scholar] [CrossRef]
- Hatanaka, N.; Xu, B.; Yasugi, M.; Morino, H.; Tagishi, H.; Miura, T.; Shibata, T.; Yamasaki, S. Chlorine dioxide is a more potent antiviral agent against SARS-CoV-2 than sodium hypochlorite. J. Hosp. Infect. 2021, 118, 20–26. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. A critical review over the electrochemical disinfection of bacteria in synthetic and real wastewaters using a boron-doped diamond anode. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100926. [Google Scholar] [CrossRef]
- Sales Monteiro, M.K.; Sales Monteiro, M.M.; de Melo Henrique, A.M.; Llanos, J.; Saez, C.; Dos Santos, E.V.; Rodrigo, M.A. A review on the electrochemical production of chlorine dioxide from chlorates and hydrogen peroxide. Curr. Opin. Electrochem. 2021, 27, 100685. [Google Scholar] [CrossRef]
- Isaza Ferro, E.; Perrin, J.; Dawson, O.G.J.; Vuorinen, T. Tertiary amine-catalyzed generation of chlorine dioxide from hypochlorous acid and chlorite ions. Wood Sci. Technol. 2021, 55, 67–81. [Google Scholar] [CrossRef]
- Qi, M.H.; Yi, T.; Mo, Q.; Huang, L.J.; Zhao, H.Y.; Xu, H.; Huang, C.X.; Wang, S.F.; Liu, Y.; Hui, Z. Preparation of High-Purity Chlorine Dioxide by Combined Reduction. Chem. Eng. Technol. 2020, 43, 1850–1858. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Hsieh, Y.-H.; Yang, C.-L.; Chang, C.-Y.; You, S.-H. Using membrane electrolysis method to generate chlorine dioxide. In Proceedings of the International Conference on Environment Science and Engineering (ICESE), Bali, Indonesia, 1–3 April 2011; pp. 33–36. [Google Scholar]
- Pillai, K.C.; Kwon, T.O.; Park, B.B.; Moon, I.S. Using RuO2 anode for chlorine dioxide production in an un-divided electrochemical cell. Water Sci. Technol. 2010, 61, 2151–2160. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Lee, H.K. Manufacture of chlorine dioxide from sodium chlorate: State of the art. J. Ind. Eng. Chem. 2005, 11, 330–346. [Google Scholar]
- Pillai, K.C.; Kwon, T.O.; Park, B.B.; Moon, I.S. Studies on process parameters for chlorine dioxide production using IrO2 anode in an un-divided electrochemical cell. J. Hazard. Mater. 2009, 164, 812–819. [Google Scholar] [CrossRef]
- Wu, C.T.; Chang, C.Y.; Li, Y.Y.; Kuan, Y.L.; Lin, P.H. An efficiency analysis for the production of chlorine dioxide by the electrolysis of brine in seawater desalination plants. Water Qual. Res. J. 2019, 54, 127–133. [Google Scholar] [CrossRef]
- Lee, J.; Lee, N.T.; Ryu, J.H. Effect of various environmental factors such as concentration of NaClO2, relative humidity, temperature, and time on the production of gaseous chlorine dioxide. Korean J. Food Sci. Technol. 2019, 51, 404–409. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, B. Development of System on the Sustained Production of Chlorine Dioxide Using Polymer Hydrogels. Korean Chem. Eng. Res. 2012, 50, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Deshwal, B.R.; Lee, H.K. Manufacture of chlorine dioxide from sodium chlorite: Process chemistry. J. Ind. Eng. Chem. 2005, 11, 125–136. [Google Scholar]
- Deshwal, B.R.; Lee, H.K. Kinetics and mechanism of chloride based chlorine dioxide generation process from acidic sodium chlorate. J. Hazard. Mater. 2004, 108, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Ni, Y. Using hydrogen peroxide in a methanol-based chlorine dioxide generation process. Ind. Eng. Chem. Res. 1999, 38, 3319–3323. [Google Scholar] [CrossRef]
- Ni, Y.H.; Wang, X.L. Mechanism and kinetics of chlorine dioxide reaction with hydrogen peroxide under acidic conditions. Can. J. Chem. Eng. 1997, 75, 31–36. [Google Scholar] [CrossRef]
- Burke, M.; Tenney, J.; Indu, B.; Hoq, M.F.; Carr, S.; Ernst, W.R. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide. Ind. Eng. Chem. Res. 1993, 32, 1449–1456. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Moratalla, Á.; Sáez, C.; Santos, E.V.D.; Rodrigo, M.A. Production of Chlorine Dioxide Using Hydrogen Peroxide and Chlorates. Catalysts 2021, 11, 1478. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, Y.; Jiang, Y.; Zhang, L. A clean production process of sodium chlorite from sodium chlorate. J. Clean. Prod. 2007, 15, 920–926. [Google Scholar] [CrossRef]
- Costa, M.L.; Cowley, G.; Pu, C. Production of Alkali metal Chlorite, e.g., Sodium Chlorite, as Precursor of Chlorine Dioxide for Water Treatment, by Generating Chlorine Dioxide by Reducing Chlorate Ions with Methanol to Chlorine Dioxide in Aqueous Acid Reaction Medium. U.S. Patent 4087515A, 30 June 1976. [Google Scholar]
- Endrodi, B.; Stojanovic, A.; Cuartero, M.; Simic, N.; Wildlock, M.; de Marco, R.; Crespo, G.A.; Cornell, A. Selective Hydrogen Evolution on Manganese Oxide Coated Electrodes: New Cathodes for Sodium Chlorate Production. ACS Sustain. Chem. Eng. 2019, 7, 12170–12178. [Google Scholar] [CrossRef]
- Macounova, K.M.; Simic, N.; Ahlberg, E.; Krtil, P. Electrocatalytic Aspects of the Chlorate Process: A Voltammetric and DEMS Comparison of RuO2 and DSA Anodes. J. Electrochem. Soc. 2018, 165, E751–E758. [Google Scholar] [CrossRef]
- Endrodi, B.; Sandin, S.; Smulders, V.; Simic, N.; Wildlock, M.; Mul, G.; Mei, B.T.; Cornell, A. Towards sustainable chlorate production: The effect of permanganate addition on current efficiency. J. Clean. Prod. 2018, 182, 529–537. [Google Scholar] [CrossRef]
- Wanngard, J.; Wildlock, M. The catalyzing effect of chromate in the chlorate formation reaction. Chem. Eng. Res. Des. 2017, 121, 438–447. [Google Scholar] [CrossRef]
- Endrodi, B.; Simic, N.; Wildlock, M.; Cornell, A. A review of chromium (VI) use in chlorate electrolysis: Functions, challenges and suggested alternatives. Electrochim. Acta 2017, 234, 108–122. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef] [PubMed]
- Llanos, J.; Moraleda, I.; Sáez, C.; Rodrigo, M.A.; Cañizares, P. Electrochemical production of perchlorate as an alternative for the valorization of brines. Chemosphere 2019, 220, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrochemical production of perchlorates using conductive diamond electrolyses. Chem. Eng. J. 2011, 166, 710–714. [Google Scholar] [CrossRef]
- Neodo, S.; Rosestolato, D.; Ferro, S.; De Battisti, A. On the electrolysis of dilute chloride solutions: Influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochim. Acta 2012, 80, 282–291. [Google Scholar] [CrossRef]
- Selcuk, H.; Anderson, M.A. Effect of pH, charge separation and oxygen concentration in photoelectrocatalytic systems: Active chlorine production and chlorate formation. Desalination 2005, 176, 219–227. [Google Scholar] [CrossRef]
- Valim, R.B.; Reis, R.M.; Castro, P.S.; Lima, A.S.; Rocha, R.S.; Bertotti, M.; Lanza, M.R.V. Electrogeneration of hydrogen peroxide in gas diffusion electrodes modified with tert-butyl-anthraquinone on carbon black support. Carbon 2013, 61, 236–244. [Google Scholar] [CrossRef]
- Fernando Perez, J.; Llanos, J.; Saez, C.; Lopez, C.; Canzares, P.; Andres Rodrigo, M. Towards the scale up of a pressurized-jet microfluidic flow-through reactor for cost-effective electro-generation of H2O2. J. Clean. Prod. 2019, 211, 1259–1267. [Google Scholar] [CrossRef]
- Pérez, J.F.; Galia, A.; Rodrigo, M.A.; Llanos, J.; Sabatino, S.; Sáez, C.; Schiavo, B.; Scialdone, O. Effect of pressure on the electrochemical generation of hydrogen peroxide in undivided cells on carbon felt electrodes. Electrochim. Acta 2017, 248, 169–177. [Google Scholar] [CrossRef]
- Pérez, J.F.; Llanos, J.; Sáez, C.; López, C.; Cañizares, P.; Rodrigo, M.A. The jet aerator as oxygen supplier for the electrochemical generation of H2O2. Electrochim. Acta 2017, 246, 466–474. [Google Scholar] [CrossRef]
- Perez, J.F.; Llanos, J.; Saez, C.; Lopez, C.; Canizares, P.; Rodrigo, M.A. Electrochemical jet-cell for the in-situ generation of hydrogen peroxide. Electrochem. Commun. 2016, 71, 65–68. [Google Scholar] [CrossRef]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.F.; Sáez, C.; Llanos, J.; Cañizares, P.; López, C.; Rodrigo, M.A. Improving the Efficiency of Carbon Cloth for the Electrogeneration of H2O2: Role of Polytetrafluoroethylene and Carbon Black Loading. Ind. Eng. Chem. Res. 2017, 56, 12588–12595. [Google Scholar] [CrossRef]
- Perry, S.C.; Mavrikis, S.; Wang, L.; Ponce de León, C. Future perspectives for the advancement of electrochemical hydrogen peroxide production. Curr. Opin. Electrochem. 2021, 30, 100792. [Google Scholar] [CrossRef]
- Na, J.; Seo, B.; Kim, J.; Lee, C.W.; Lee, H.; Hwang, Y.J.; Min, B.K.; Lee, D.K.; Oh, H.S.; Lee, U. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 2019, 10, 5193. [Google Scholar] [CrossRef]
- Moratalla, Á.; Araújo, D.M.; Moura, G.O.M.A.; Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Pressurized electro-Fenton for the reduction of the environmental impact of antibiotics. Sep. Purif. Technol. 2021, 276, 119398. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]








| Test | Volume/mL | TªReactor/°C | mmol H2O2 /mmolClO3− Ratio | ||
|---|---|---|---|---|---|
| H2O2 (3.3 g L−1) | NaClO3 (5 g L−1) | H2SO4 (5.0 M) | |||
| Q1 | 6 | 10 | 25 | 50 | 1.2 |
| Q2 | |||||
| Q3 | |||||
| Q4 | |||||
| Q5 | 4 | 10 | 25 | 83 | 0.83 |
| Q6 | |||||
| Q7 | |||||
| Q8 | |||||
| Q9 | 0.5 | 5 | 25 | 60 | 0.2 |
| Q10 | 2.5 | 5 | 25 | 1.0 | |
| Q11 | 10 | 5 | 25 | 4.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moratalla, Á.; Monteiro, M.K.S.; Sáez, C.; Dos Santos, E.V.; Rodrigo, M.A. Full and Sustainable Electrochemical Production of Chlorine Dioxide. Catalysts 2022, 12, 315. https://doi.org/10.3390/catal12030315
Moratalla Á, Monteiro MKS, Sáez C, Dos Santos EV, Rodrigo MA. Full and Sustainable Electrochemical Production of Chlorine Dioxide. Catalysts. 2022; 12(3):315. https://doi.org/10.3390/catal12030315
Chicago/Turabian StyleMoratalla, Ángela, Mayra K. S. Monteiro, Cristina Sáez, Elisama V. Dos Santos, and Manuel A. Rodrigo. 2022. "Full and Sustainable Electrochemical Production of Chlorine Dioxide" Catalysts 12, no. 3: 315. https://doi.org/10.3390/catal12030315
APA StyleMoratalla, Á., Monteiro, M. K. S., Sáez, C., Dos Santos, E. V., & Rodrigo, M. A. (2022). Full and Sustainable Electrochemical Production of Chlorine Dioxide. Catalysts, 12(3), 315. https://doi.org/10.3390/catal12030315