Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of NiAl LDHs and Mixed Oxides
2.2. Formation of Catalytically Active Phase upon Reductive Treatment
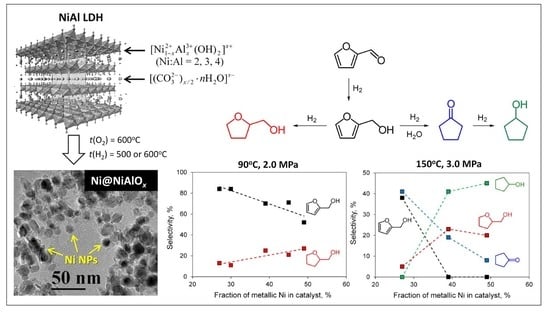
2.3. Performance of Ni@NiAlOx Catalysts in Aqueous-Phase Hydrogenation of Furfural (FAL)
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; van Bekkum, H. Fine Chemicals through Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar] [CrossRef]
- Jackson, S.D. (Ed.) Hydrogenation: Catalysts and Processes; Walter de Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Bonrath, W.; Medlock, J.; Schütz, J.; Wüstenberg, B.; Netscher, T. Hydrogenation in the Vitamins and Fine Chemicals Industry—An Overview. In Hydrogenation; Karamé, I., Ed.; IntechOpen: London, UK, 2012; pp. 69–90. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.G.; Youn, M.H.; Song, I.K. Mesoporous nickel–alumina catalysts for hydrogen production by steam reforming of liquefied natural gas (LNG). Catal. Surv. Asia 2010, 14, 1–10. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, K.; Shi, Z.; Wan, C.; Pan, J.; Li, D.; Au, C.; Jiang, L. Influence of reduction temperature on Ni particle size and catalytic performance of Ni/Mg(Al)O catalyst for CO2 reforming of CH4. Int. J. Hydrog. Energy 2020, 45, 2794–2807. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Stepinska, N.; Chalupka, K.; Nowosielska, M.; Maniukiewicz, W.; Rogowski, J.; Goswami, N.; Vasilev, K.; Szynkowska, M.I. Effect of the support composition on catalytic and physicochemical properties of Ni catalysts in oxy-steam reforming of methane. Catal. Today 2021, 364, 46–60. [Google Scholar] [CrossRef]
- Abdelsadek, Z.; Holgado, J.P.; Halliche, D.; Caballero, A.; Cherifi, O.; Gonzalez-Cortes, S.; Masset, P.J. Examination of the deactivation cycle of NiAl- and NiMgAl-hydrotalcite derived catalysts in the dry reforming of methane. Catal. Lett. 2021, 151, 2696–2715. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Abild-Pedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B.; Rostrup-Nielsen, J.R.; et al. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic conversion of methane to synthesis gas by partial oxidation and CO2 reforming. Adv. Catal. 2004, 48, 297–345. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, A.P.; Remediakis, I.N.; Bengaard, H.; Nørskov, J.K. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Chen, I.; Lin, S.-Y.; Shiue, D.-W. Calcination of nickel/alumina catalysts. Ind. Eng. Chem. Res. 1988, 27, 926–929. [Google Scholar] [CrossRef]
- Ye, R.-P.; Liao, L.; Reina, T.R.; Liu, J.; Chevella, D.; Jin, Y.; Fan, M.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2021, 285, 119151. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. (Eds.) Layered Double Hydroxides; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Rives, V. (Ed.) Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Sileo, E.E.; Jobbágy, M.; Paiva-Santos, C.O.; Regazzoni, A.E. Thermal decomposition of crystalline NiII−CrIII layered Double hydroxide: A structural study of the segregation process. J. Phys. Chem. B 2005, 109, 10137–10141. [Google Scholar] [CrossRef] [PubMed]
- Belskaya, O.B.; Leont’eva, N.N.; Gulyaeva, T.I.; Cherepanova, S.V.; Talzi, V.P.; Drozdov, V.A.; Likholobov, V.A. Influence of a doubly charged cation nature on the formation and properties of mixed oxides MAlOx (M = Mg2+, Zn2+, Ni2+) obtained from the layered hydroxide precursors. Russ. Chem. Bull. 2013, 62, 2349–2361. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- Holgado, M.J.; Rives, V.; San Román, M.S. Characterization of Ni–Mg–Al mixed oxides and their catalytic activity in oxidative dehydrogenation of n-butane and propene. Appl. Catal. A 2001, 214, 219–228. [Google Scholar] [CrossRef]
- Mas, V.; Dieuzeide, M.L.; Jobbágy, M.; Baronetti, G.; Amadeo, N.; Laborde, M. Ni(II)-Al(III) layered double hydroxide as catalyst precursor for ethanol steam reforming: Activation treatments and kinetic studies. Catal. Today 2008, 133–135, 319–323. [Google Scholar] [CrossRef]
- Tichit, D.; Medina, F.; Coq, B.; Dutartre, R. Activation under oxidizing and reducing atmospheres of Ni-containing layered double hydroxides. Appl. Catal. A 1997, 159, 241–258. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Bezdička, P.; Jirátová, K.; Obalová, L.; Pacultová, K.; Bastl, Z.; Grygar, T. Effect of hydrothermal treatment on properties of Ni–Al layered double hydroxides and related mixed oxides. J. Solid State Chem. 2009, 182, 27–36. [Google Scholar] [CrossRef]
- Abelló, S.; Verboekend, D.; Bridier, B.; Pérez-Ramírez, J. Activated takovite catalysts for partial hydrogenation of ethyne, propyne, and propadiene. J. Catal. 2008, 259, 85–95. [Google Scholar] [CrossRef]
- Song, Y.; Beaumont, S.K.; Zhang, X.; Wilson, K.; Lee, A.F. Catalytic applications of layered double hydroxides in biomass valorisation. Curr. Opin. Green Sustain. Chem. 2020, 22, 29–38. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Z.; Zhou, S.; Wei, M. Perspectives on multifunctional catalysts derived from layered double hydroxides toward upgrading reactions of biomass resources. ACS Catal. 2021, 11, 6440–6454. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Sun, Z.; Yuan, T.-Q. Recent advances in the catalytic upgrading of biomass platform chemicals via hydrotalcite-derived metal catalysts. Trans. Tianjin Univ. 2022, 28, 89–111. [Google Scholar] [CrossRef]
- Zelinsky, N.; Kommarewsky, W. Über die katalytischen Wirkungen des nickelierten Tonerde-Hydrats. Ber. Dtsch. Chem. Ges. 1924, 57, 667–669. [Google Scholar] [CrossRef]
- Puxley, D.C.; Kitchener, I.J.; Komodromos, C.; Parkyns, N.D. The effect of preparation method upon the structures, stability and metal/support interactions in nickel/alumina catalysts. Stud. Surf. Sci. Catal. 1983, 16, 237–271. [Google Scholar] [CrossRef]
- Rudolf, C.; Dragoi, B.; Ungureanu, A.; Chirieac, A.; Royer, S.; Nastro, A.; Dumitriu, E. NiAl and CoAl materials derived from takovite-like LDHs and related structures as efficient chemoselective hydrogenation catalysts. Catal. Sci. Technol. 2014, 4, 179–189. [Google Scholar] [CrossRef]
- Han, J.; Jia, H.; Yang, Z.; Fan, Q.; Zhang, F. Confined hexahedral nickel nanoparticle catalyst for catalytic hydrogenation reaction. J. Mater. Sci. 2018, 53, 4884–4896. [Google Scholar] [CrossRef]
- Li, Z.; Shi, R.; Zhao, J.; Zhang, T. Ni-based catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 reduction under flow-type system. Nano Res. 2021, 14, 4828–4832. [Google Scholar] [CrossRef]
- Pothu, R.; Gundeboyina, R.; Boddula, R.; Perugopu, V.; Ma, J. Recent advances in biomass-derived platform chemicals to valeric acid synthesis. New J. Chem. 2022, 46, 5907–5921. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Salanov, A.N.; Serkova, A.N.; Likholobov, V.A. SEM study of the surface morphology and chemical composition of the MgAl- and MgGa-layered hydroxides in different steps of platinum catalysts Pt/Mg(Al, Ga)Ox synthesis. Appl. Clay Sci. 2018, 157, 267–273. [Google Scholar] [CrossRef]
- Jinesh, C.M.; Antonyraj, C.A.; Kannan, S. Allylbenzene isomerisation over as-synthesized MgAl and NiAl containing LDHs: Basicity-activity relationships. Appl. Clay Sci. 2010, 48, 243–249. [Google Scholar] [CrossRef]
- Titulaer, M.K.; Jansen, J.B.H.; Geus, J.W. The quantity of reduced nickel in synthetic takovite: Effects of preparation conditions and calcination temperature. Clays Clay Miner. 1994, 42, 249–258. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belskaya, O.B.; Zaikovskii, V.I.; Gulyaeva, T.I.; Talsi, V.P.; Trubina, S.V.; Kvashnina, K.O.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Likholobov, V.A. The effect of Pd(II) chloride complexes anchoring on the formation and properties of Pd/MgAlOx catalysts. J. Catal. 2020, 392, 108–118. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Approaches to the synthesis of Pd/C catalysts with controllable activity and selectivity in hydrogenation reactions. Catal. Today 2020, 357, 152–165. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Talsi, V.P.; Gulyaeva, T.I.; Trenikhin, M.V.; Belskaya, O.B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: Effect of the support on the reaction routes. React. Kinet. Mech. Catal. 2019, 126, 811–827. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Talsi, V.P.; Likholobov, V.A. Mechanism of Pd/C-catalyzed hydrogenation of furfural under hydrothermal conditions. J. Catal. 2020, 389, 721–734. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Jia, X.; Du, Z.; Duan, Y.; Xu, J. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol on alkaline earth metal modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Rietveld, H.M. The Rietveld method: A retrospection. Z. Kristallogr. 2010, 225, 545–547. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joiner, L.G.; Halenda, P.H. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]











| Calculated Ni/Al Atomic Ratio | Content, wt % 1 | Measured Ni/Al Atomic Ratio 1 | c, Å 2 | a, Å 2 | Lc, nm 2 | La, nm 2 | ||
|---|---|---|---|---|---|---|---|---|
| Ni | Al | O | ||||||
| 2 | 53.3 | 12.2 | 34.5 | 2.1 | 22.745 | 3.021 | 48.8 | 77.9 |
| 3 | 62.2 | 8.5 | 29.3 | 3.0 | 23.232 | 3.043 | 71.1 | 116.7 |
| 4 | 64.2 | 6.9 | 28.9 | 4.1 | 23.478 | 3.054 | 48.6 | 102.6 |
| Sample | Phase | Fraction, % | Unit Cell Parameters, nm | CSR, nm 1 |
|---|---|---|---|---|
| Ni@NiAlOx-2-500 | Ni | 27 | 0.3532 | 5.2 |
| NiO | 73 | 0.4135 | 2.4 | |
| Ni@NiAlOx-3-500 | Ni | 30 | 0.3538 | 3.6 |
| NiO | 70 | 0.4156 | 3.7 | |
| Ni@NiAlOx-4-500 | Ni | 39 | 0.3533 | 5.7 |
| NiO | 61 | 0.4136 | 2.5 | |
| Ni@NiAlOx-3-600 | Ni | 45 | 0.3529 | 10.9 |
| NiO | 55 | 0.4122 | 3.4 | |
| Ni@NiAlOx-4-600 | Ni | 49 | 0.3528 | 9.7 |
| NiO | 51 | 0.4116 | 3.0 |
| Sample | NiO | Ni Metal | ||||||
| Fraction, % | Coordination Sphere | R, Å 1 | N 2 | Fraction, % | Coordination Sphere | R, Å 1 | N 2 | |
| Ni@NiAlOx-2-500 | 90 | Ni–O | 2.09 | 6 | 10 | Ni–Ni | 2.48 | 12 |
| Ni–Ni | 2.97 | 12 | ||||||
| Fit = 2.6 | ||||||||
| Ni@NiAlOx-3-500 | 70 | Ni–O | 2.10 | 6 | 30 | Ni–Ni | 2.47 | 12 |
| Ni–Ni | 2.97 | 12 | ||||||
| Fit = 2.9 | ||||||||
| Ni@NiAlOx-4-500 | 38 | Ni–O | 2.13 | 6 | 62 | Ni–Ni | 2.48 | 12 |
| Ni–Ni | 2.99 | 12 | ||||||
| Fit = 1.6 | ||||||||
| Ni@NiAlOx-3-600 | 35 | Ni–O | 2.13 | 6 | 65 | Ni–Ni | 2.48 | 12 |
| Ni–Ni | 3.00 | 12 | ||||||
| Fit = 1.1 | ||||||||
| Ni@NiAlOx-4-600 | 30 | Ni–O | 2.13 | 6 | 70 | Ni–Ni | 2.49 | 12 |
| Ni–Ni | 3.00 | 12 | ||||||
| Fit = 2.3 | ||||||||
| Crystallographic data for NiO | ||||||||
| Coordination spheres | R, Å 1 | N 2 | ||||||
| Ni–O | 2.08 | 6 | ||||||
| Ni–Ni | 2.94 | 12 | ||||||
| Crystallographic data for Ni metal | ||||||||
| Coordination sphere | R, Å 1 | N 2 | ||||||
| Ni–Ni | 2.49 | 12 | ||||||
| 3.52 | 6 | |||||||
| 4.32 | 24 | |||||||
| Catalyst 1 | Conversion of FAL, % 2 | Selectivity, % 3 | Reaction Rate, (mmol H2)∙min−1 4 | |
|---|---|---|---|---|
| FOL | THFOL | |||
| Ni@NiAlOx-2-500 | 39 | 84 | 13 | 22.7 |
| Ni@NiAlOx-3-500 | 43 | 84 | 11 | 22.1 |
| Ni@NiAlOx-4-500 | 93 | 70 | 25 | 38.5 |
| Ni@NiAlOx-3-600 | 75 | 71 | 21 | 22.1 |
| Ni@NiAlOx-4-600 | 98 | 52 | 27 | 33.0 |
| NiAlOx-3 (unreduced) | 0 | – | – | – |
| Catalyst 1 | Conversion of FAL, % 2 | Selectivity, % 3 | |||
|---|---|---|---|---|---|
| FOL | THFOL | CPONE | CPOL | ||
| Ni@NiAlOx-2-500 | 67 | 38 | 5 | 41 | 0 |
| Ni@NiAlOx-4-500 | >99 | 0 | 23 | 19 | 41 |
| Ni@NiAlOx-2-600 4 | >99 | 2 | 33 | 43 | 8 |
| Ni@NiAlOx-4-600 4 | >99 | 0 | 20 | 8 | 45 |
| 10%Ni/γ-Al2O3 | 37 | 4 | 7 | 59 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belskaya, O.B.; Mironenko, R.M.; Gulyaeva, T.I.; Trenikhin, M.V.; Muromtsev, I.V.; Trubina, S.V.; Zvereva, V.V.; Likholobov, V.A. Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation. Catalysts 2022, 12, 598. https://doi.org/10.3390/catal12060598
Belskaya OB, Mironenko RM, Gulyaeva TI, Trenikhin MV, Muromtsev IV, Trubina SV, Zvereva VV, Likholobov VA. Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation. Catalysts. 2022; 12(6):598. https://doi.org/10.3390/catal12060598
Chicago/Turabian StyleBelskaya, Olga B., Roman M. Mironenko, Tatiana I. Gulyaeva, Mikhail V. Trenikhin, Ivan V. Muromtsev, Svetlana V. Trubina, Valentina V. Zvereva, and Vladimir A. Likholobov. 2022. "Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation" Catalysts 12, no. 6: 598. https://doi.org/10.3390/catal12060598
APA StyleBelskaya, O. B., Mironenko, R. M., Gulyaeva, T. I., Trenikhin, M. V., Muromtsev, I. V., Trubina, S. V., Zvereva, V. V., & Likholobov, V. A. (2022). Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation. Catalysts, 12(6), 598. https://doi.org/10.3390/catal12060598