Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Composite Photocatalysts
2.2. CO2 Reduction Activity of Photocatalysts
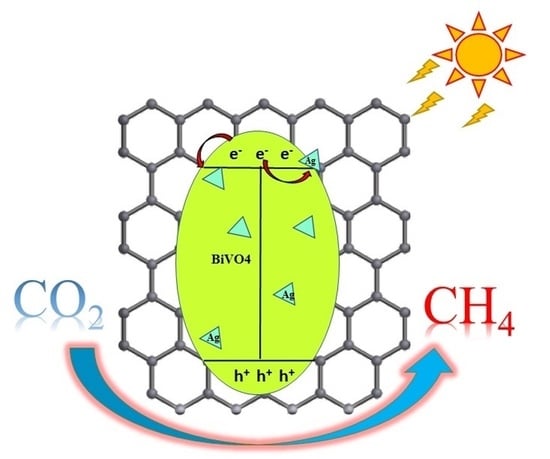
2.3. Mechanism of the CO2 Reduction Reaction
3. Experiment
3.1. Materials
3.2. Preparation of Graphene/BiVO4 Catalyst and Ag Nanomaterials
3.3. Fabrication of Ag/Graphene/BiVO4 Composites
3.4. XRD, SEM, TEM/EDS, UV–Vis, PL and XPS
3.5. Photocatalytic Reduction of CO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Wilberforce, T.; Baroutaji, A.; Soudan, B.; Al-Alami, A.H.; Olabi, A.G. Outlook of carbon capture technology and challenges. Sci. Total Environ. 2019, 657, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artz, J.; Müller, T.E.; Thenert, K.M.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Han, B.; Song, J.; Liang, S.; Chen, W.; Deng, H.; Ou, X.; Xu, Y.-J.; Lin, Z. Hierarchical NiCo2O4 hollow nanocages for photoreduction of diluted CO2: Adsorption and active sites engineering. Appl. Catal. B Environ. 2020, 260, 118208. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Cao, Y. Synthesis of palladium-modified MnS photocatalysts with enhanced photocatalytic activity in the photoreduction of CO2 to CH4. Appl. Surf. Sci. 2021, 541, 148519. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Yang, Y.; Wu, J.; Shen, J.; Liu, X.; Sun, A.; Liu, Z. In-situ construction of urchin-like hierarchical g-C3N4/NiAl-LDH hybrid for efficient photoreduction of CO2. Mater. Lett. 2020, 268, 127560. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, R.; Wang, J.; Cui, C.; Wang, J.; Li, Z. A novel hollow-hierarchical structured Bi2WO6 with enhanced photocatalytic activity for CO2 photoreduction. J. Colloid Interface Sci. 2018, 523, 151–158. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Bian, J.; Sun, N.; Sun, J.; Chen, L.; Li, Z.; Jing, L. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118849. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, C.; Li, R.; Wang, Y.; Fan, C. Novel Bi4Ti3O12 hollow-spheres with highly-efficient CO2 photoreduction activity. Inorg. Chem. Commun. 2020, 116, 107931. [Google Scholar] [CrossRef]
- Huang, Y.F.; Liao, K.W.; Fahmi, F.R.Z.; Modak, V.A.; Tsai, S.H.; Ke, S.W.; Wang, C.H.; Chen, L.C.; Chen, K.H. Thickness-Dependent Photocatalysis of Ultra-Thin MoS2 Film for Visible-Light-Driven CO2 Reduction. Catalysts 2021, 11, 1295. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.-X.; Hwang, Y.-T.; Lin, Y.-C.; Wu, R.-J. Fuel generation through photoreduction of CO2 on novel Cu/BiVO4. Mater. Res. Bull. 2020, 130, 110955. [Google Scholar] [CrossRef]
- Zhu, Z.; Hwang, Y.T.; Liang, H.C.; Wu, R.J. Prepared Pd/MgO/BiVO4 composite for photoreduction of CO2 to CH4. J. Chin. Chem. Soc. 2021, 68, 1897–1907. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Wang, Y.-F.; Li, Y.-Y.; Zhang, L.; Yao, H.-C.; Li, Z.-J. Enhanced photocatalytic CO2 reduction activity of Z-scheme CdS/BiVO4 nanocomposite with thinner BiVO4 nanosheets. J. CO2 Util. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Rather, R.A.; Khan, M.; Lo, I.M. High charge transfer response of g-C3N4/Ag/AgCl/BiVO4 microstructure for the selective photocatalytic reduction of CO2 to CH4 under alkali activation. J. Catal. 2018, 366, 28–36. [Google Scholar] [CrossRef]
- Han, Q.; Li, L.; Gao, W.; Shen, Y.; Wang, L.; Zhang, Y.; Wang, X.; Shen, Q.; Xiong, Y.; Zhou, Y.; et al. Elegant Construction of ZnIn2S4/BiVO4 Hierarchical Heterostructures as Direct Z-Scheme Photocatalysts for Efficient CO2 Photoreduction. ACS Appl. Mater. Interfaces 2021, 13, 15092–15100. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Hsu, H.-L.; Juang, T.-Y.; Chen, C.-P.; Hsieh, C.-M.; Yang, C.-C.; Huang, C.-L.; Jeng, R.-J. Enhanced efficiency of organic and perovskite photovoltaics from shape-dependent broadband plasmonic effects of silver nanoplates. Sol. Energy Mater. Sol. Cells 2015, 140, 224–231. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Loh, K.P. Graphene Photonics, Plasmonics, and Broadband Optoelectronic Devices. ACS Nano 2012, 6, 3677–3694. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ko, S.-J.; Choi, Y.; Joo, P.; Kim, T.; Lee, B.R.; Jung, J.-W.; Choi, H.J.; Cha, M.; Jeong, J.-R.; et al. Versatile surface plasmon resonance of carbon-dot-supported silver nanoparticles in polymer optoelectronic devices. Nat. Photonics 2013, 7, 732–738. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced Conversion of Silver Nanospheres to Nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ge, J.; Pham, T.; Goebl, J.; Hu, Y.; Lu, Z.; Yin, Y. Reconstruction of Silver Nanoplates by UV Irradiation: Tailored Optical Properties and Enhanced Stability. Angew. Chem. Int. Ed. 2009, 48, 3516–3519. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hsu, M.-S.; Hsu, Y.-C.; Lo, C.-W.; Huang, C.-L. A Facile Method to Obtain Highly Stable Silver Nanoplate Colloids with Desired Surface Plasmon Resonance Wavelengths. J. Phys. Chem. C 2010, 114, 6222–6227. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, Y.-C.; Chung, C.-L.; Wu, R.-J.; Huang, C.-L. A novel composite of triangular silver nanoplates on BiVO4 for gaseous formaldehyde degradation. Appl. Surf. Sci. 2021, 543, 148784. [Google Scholar] [CrossRef]
- Lin, W.-D.; Liao, C.-T.; Chang, T.-C.; Chen, S.-H.; Wu, R.-J. Humidity sensing properties of novel graphene/TiO2 composites by sol–gel process. Sens. Actuators B Chem. 2015, 209, 555–561. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J. Synthesis and characterization of Ag/BiVO4 composite photocatalyst. Appl. Surf. Sci. 2010, 256, 3224–3227. [Google Scholar] [CrossRef]
- Guo, R.; Yan, A.; Xu, J.; Xu, B.; Li, T.; Liu, X.; Yi, T.; Luo, S. Effects of morphology on the visible-light-driven photocatalytic and bactericidal properties of BiVO4/CdS heterojunctions: A discussion on photocatalysis mechanism. J. Alloys Compd. 2020, 817, 153246. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Zhang, J. Mo-doped BiVO4 and graphene nanocomposites with enhanced photoelectrochemical performance for aptasensing of streptomycin. Carbon 2017, 120, 194–202. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.M.; Rao, C.N.R. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega 2017, 2, 2740–2748. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Wang, P.; Dai, Y.; Huang, B. Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl. Surf. Sci. 2018, 456, 493–500. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Christopher, P.; Moskovits, M. Hot Charge Carrier Transmission from Plasmonic Nanostructures. Annu. Rev. Phys. Chem. 2017, 68, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Lai, Y.-S.; Tsai, C.-M.; Kong, Y.-T.; Lee, C.-I.; Huang, C.-L. One-Pot Synthesis of Monodispersed Silver Nanodecahedra with Optimal SERS Activities Using Seedless Photo-Assisted Citrate Reduction Method. J. Phys. Chem. C 2012, 116, 24292–24300. [Google Scholar] [CrossRef]
- Xie, Z.-X.; Tzeng, W.-C.; Huang, C.-L. One-Pot Synthesis of Icosahedral Silver Nanoparticles by Using a Photoassisted Tartrate Reduction Method under UV Light with a Wavelength of 310 nm. ChemPhysChem 2016, 17, 2551–2557. [Google Scholar] [CrossRef]
- Ciou, S.-H.; Cao, Y.-W.; Huang, H.-C.; Su, D.-Y.; Huang, C.-L. SERS Enhancement Factors Studies of Silver Nanoprism and Spherical Nanoparticle Colloids in The Presence of Bromide Ions. J. Phys. Chem. C 2009, 113, 9520–9525. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]















| Year/Reference | Photocatalysts /Light Source | Rate of CH4 (μmol g−1 h−1) | Quantum Efficiency (%) |
|---|---|---|---|
| 2018 [18] | CdS/BiVO4 300 W xenon lamp | 2.1 | -- |
| 2018 [19] | g-C3N4/Ag/AgCl/BiVO4 64 W fluorescent lamps | 5.3 | -- |
| 2020 [16] | 0.5% Cu/BiVO4 400 W Hg light | 7.4 | 0.19 |
| 2021 [17] | 0.3% Pd/MgO/BiVO4 400 W Hg light | 12.8 | 0.34 |
| 2021 [20] | ZnIn2S4/BiVO4 | 0.3 | -- |
| 2021 [15] | Ultrathin MoS2 film | 1.38 nmol/cm2 | 0.00007 |
| This work | 0.003%AgNPts/ 7% graphene/BiVO4 400 W Hg light | 18.1 | 0.49 |
| Photocatalyst | Band Gap (eV) | Valence Band (eV) | Conduction Band (eV) |
|---|---|---|---|
| BiVO4 | 2.40 | 1.50 | −0.90 |
| 7% graphene/BiVO4 | 2.40 | -- | -- |
| 0.1% TAgNPt/7% graphene /BiVO4 | 2.38 | 1.25 | −1.13 |
| 0.5 wt% I-AgNP /BiVO4 | 2.40 | -- | -- |
| Photocatalysts | Rate of CH4 (μmol g−1 h−1) |
|---|---|
| TiO2 (P25) | 0.4 |
| BiVO4 | 3.6 |
| 1 wt% graphene/BiVO4 | 4.4 |
| 2 wt% graphene/BiVO4 | 5.2 |
| 3.5 wt% graphene/BiVO4 | 5.6 |
| 5 wt% graphene/BiVO4 | 6.4 |
| 7 wt% graphene/BiVO4 | 7.7 |
| 8.5 wt% graphene/BiVO4 | 5.7 |
| 10 wt% graphene/BiVO4 | 5.3 |
| Photocatalysts | Rate of CH4 (μmol g−1 h−1) |
|---|---|
| BiVO4 | 3.6 |
| 7 wt% graphene/BiVO4 | 7.7 |
| 0.001 wt% TAgNPts/7 wt% graphene/BiVO4 | 12.7 |
| 0.003 wt% TAgNPts/7 wt% graphene/BiVO4 | 18.1 |
| 0.0075 wt% TAgNPts/7 wt% graphene/BiVO4 | 8.8 |
| 0.003 wt% D-AgNPs/7 wt% graphene/BiVO4 | 8.4 |
| 0.003 wt% I-AgNPs/7 wt% graphene/BiVO4 | 7.0 |
| 0.5 wt% I-AgNPts/7 wt% graphene/BiVO4 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Jiang, B.-X.; Wu, R.-J.; Huang, C.-L.; Chang, Y. Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4. Catalysts 2022, 12, 750. https://doi.org/10.3390/catal12070750
Zhu Z, Jiang B-X, Wu R-J, Huang C-L, Chang Y. Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4. Catalysts. 2022; 12(7):750. https://doi.org/10.3390/catal12070750
Chicago/Turabian StyleZhu, Zhen, Bo-Xun Jiang, Ren-Jang Wu, Cheng-Liang Huang, and You Chang. 2022. "Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4" Catalysts 12, no. 7: 750. https://doi.org/10.3390/catal12070750
APA StyleZhu, Z., Jiang, B. -X., Wu, R. -J., Huang, C. -L., & Chang, Y. (2022). Photoreduction of CO2 into CH4 Using Novel Composite of Triangular Silver Nanoplates on Graphene-BiVO4. Catalysts, 12(7), 750. https://doi.org/10.3390/catal12070750