The Effect of Chlorine Modification of Precipitated Iron Catalysts on Their Fischer–Tropsch Synthesis Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Freshly Prepared Catalysts
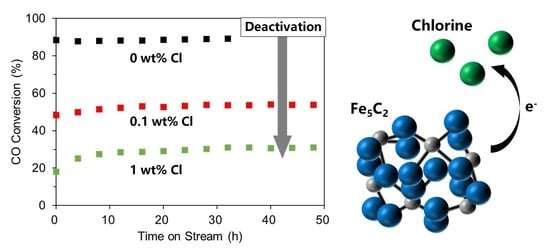
2.2. Fischer–Tropsch Synthesis Performances of the Precipitated Iron Catalysts Modified with Chlorine
2.3. Phase Change during Fischer–Tropsch Synthesis and the Reduction Properties of the Precipitated Iron Catalysts with Chlorine
2.4. Chlorine Adsorption State of the Precipitated Iron Catalysts with Chlorine
3. Materials and Methods
3.1. Materials
3.2. Preparation and Chlorine Modification of the Precipitated Iron Catalysts
3.3. Catalyst Characterization
3.4. Fischer–Tropsch Synthesis Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer-Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhou, J.; Zhang, B. Deactivation model of Fischer-Tropsch synthesis over an Fe-Cu-K commercial catalyst. Appl. Catal. A Gen. 1997, 161, 137–151. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Pendyala, V.R.R.; Hopps, S.G.; Thomas, G.A.; Hamdeh, H.H.; MacLennan, A.; Hu, Y.; Davis, B.H. Fischer–Tropsch synthesis: Effect of ammonia in syngas on the Fischer–Tropsch synthesis performance of a precipitated iron catalyst. J. Catal. 2015, 326, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Shafer, W.D.; Hamdeh, H.H.; Hopps, S.D.; Pendyala, V.R.R.; Hu, Y.; Xiao, Q.; Davis, B.H. Effect of H2S in syngas on the Fischer–Tropsch synthesis performance of a precipitated iron catalyst. Appl. Catal. A Gen. 2016, 513, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Tijmensen, M.; Faaij, A.; Hamelinck, C.N.; Hardeveld, M. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Faaij, A.P.C.; Uil, H.D.; Boerrigter, H. Production of FT transportation fuels from biomass; technical options, process analysis and optimisation, and development potential. Energy 2004, 29, 1743–1771. [Google Scholar] [CrossRef]
- Steen, E.V.; Claeys, M. Fischer-Tropsch Catalysts for the Biomass-to-Liquid (BTL)-Process. Chem. Eng. Technol. 2010, 31, 655–666. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Kang, J.; Sparks, D.E.; Davis, B.H. Fischer–Tropsch synthesis. Effect of alkali, bicarbonate and chloride addition on activity and selectivity. Catal. Today 2013, 215, 73–79. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Thomas, G.A.; Shafer, W.D.; Sparks, D.E.; Hamdeh, H.H.; Davis, B.H. Fischer–Tropsch Synthesis: Effects of Hydrohalic Acids in Syngas on a Precipitated Iron Catalyst. ACS Catal. 2015, 5, 3124–3136. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Jacobs, G.; Ma, W.; Sparks, D.E.; Shafer, W.D.; Khalid, S.; Xiao, Q.; Hu, Y.; Davis, B.H. Fischer–Tropsch Synthesis: XANES Investigation of Hydrogen Chloride Poisoned Iron and Cobalt-Based Catalysts at the K-Edges of Cl, Fe, and Co. Catal. Lett. 2016, 146, 1858–1866. [Google Scholar] [CrossRef]
- Ghosh, I.K.; Iqbal, Z.; van Heerden, T.; van Steen, E.; Bordoloi, A. Insights into the unusual role of chlorine in product selectivity for direct hydrogenation of CO/CO2 to short-chain olefins. Chem. Eng. J. 2021, 413, 127424. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer–Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wu, B.; Yang, Y.; Hao, Q.; Tian, L.; Xiang, H.; Li, Y. Effect of SiO2 content on iron-based catalysts for slurry Fischer–Tropsch synthesis. Fuel Process. Technol. 2008, 89, 284–291. [Google Scholar] [CrossRef]
- Suo, H.; Wang, S.; Zhang, C.; Jian, X.; Wu, B.; Yong, Y.; Xiang, H.; Li, Y.W. Chemical and structural effects of silica in iron-based Fischer–Tropsch synthesis catalysts. J. Catal. 2012, 286, 111–123. [Google Scholar] [CrossRef]
- Wu, B.; Tian, L.; Bai, L.; Zhang, Z.; Xiang, H.; Li, Y.W. Study on a new iron catalyst for slurry Fischer–Tropsch synthesis. Catal. Commun. 2004, 5, 253–257. [Google Scholar] [CrossRef]
- Chun, D.H.; Rhim, G.B.; Youn, M.H.; Deviana, D.; Lee, J.E.; Park, J.C.; Jeong, H. Brief Review of Precipitated Iron-Based Catalysts for Low-Temperature Fischer–Tropsch Synthesis. Top. Catal. 2020, 63, 793–809. [Google Scholar] [CrossRef]
- Lin, Q.; Cheng, M.; Zhang, K.; Li, W.; Wu, P.; Chang, H.; Lv, Y.; Men, Z. Development of an Iron-Based Fischer—Tropsch Catalyst with High Attrition Resistance and Stability for Industrial Application. Catalysts 2021, 11, 908. [Google Scholar] [CrossRef]
- Chang, H.; Cheng, M.; Lin, Q.; Zhu, J.; Lv, Y.; Men, Z. Effects of binder addition process parameters on physical-chemical and catalytic performance of iron-based Fischer-Tropsch (F-T) synthesis catalyst. J. China Coal Soc. 2021, 46, 7. [Google Scholar]
- Kim, Y.J.; Park, C.R. Analysis of Problematic Complexing Behavior of Ferric Chloride with N,N-Dimethylformamide Using Combined Techniques of FT-IR, XPS, and TGA/DTG. Inorg. Chem. 2002, 41, 6211–6216. [Google Scholar] [CrossRef]
- Kishi, K.; Ikeda, S. X-ray photoelectron spectroscopic study of the reaction of evaporated metal films with chlorine gas. J. Phys. Chem. 1974, 78, 754–758. [Google Scholar] [CrossRef]
- González-Carballo, J.M.; Pérez-Alonso, F.J.; García-García, F.J.; Ojeda, M.; Fierro, J.L.G.; Rojas, S. In-situ study of the promotional effect of chlorine on the Fischer–Tropsch synthesis with Ru/Al2O3. J. Catal. 2015, 332, 177–186. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Li, W.; Lv, Y.; Ma, Z.; Men, Z.; Yan, Z. Deactivation behavior investigation on commercial precipitated iron Fischer–Tropsch catalyst for long time reaction. J. Porous Mater. 2021, 29, 307–315. [Google Scholar] [CrossRef]







| Catalyst | Fe2O3:Cu:K:SiO2:Cl 1 | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| Cl0 | 100:3.55:1.90:10.28:0.08 | 140.8 | 0.51 | 12.1 |
| Cl01 | 100:3.76:2.04:11.33:0.14 | 140.6 | 0.52 | 12.0 |
| Cl1 | 100:3.53:1.85:10.33:1.00 | 134.4 | 0.50 | 12.2 |
| Cl5 | 100:3.55:1.41:10.71:4.02 | 123.0 | 0.48 | 12.6 |
| Catalyst | CO Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| CO2 | CH4 | C2~4 | C2~5 o/p 2 | C5+ | ||
| Cl0 | 89.0 | 16.4 | 3.2 | 7.3 | 1.4 | 73.1 |
| Cl01 | 53.9 | 19.4 | 4.5 | 9.7 | 2.2 | 66.4 |
| Cl1 | 31.1 | 8.7 | 4.2 | 8.7 | 1.9 | 78.4 |
| Catalyst | Fe2O3:Cl Before Reaction 1 | Fe2O3:Cl After Reaction 1 |
|---|---|---|
| Cl01 | 100:0.14 | 100:0.19 |
| Cl1 | 100:1.00 | 100:1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, X.; Wang, T.; Zhang, X.; Wei, L.; Lin, Q.; Lv, Y.; Men, Z. The Effect of Chlorine Modification of Precipitated Iron Catalysts on Their Fischer–Tropsch Synthesis Properties. Catalysts 2022, 12, 812. https://doi.org/10.3390/catal12080812
Li W, Zhang X, Wang T, Zhang X, Wei L, Lin Q, Lv Y, Men Z. The Effect of Chlorine Modification of Precipitated Iron Catalysts on Their Fischer–Tropsch Synthesis Properties. Catalysts. 2022; 12(8):812. https://doi.org/10.3390/catal12080812
Chicago/Turabian StyleLi, Weizhen, Xuebing Zhang, Tao Wang, Xiaoyu Zhang, Linlin Wei, Quan Lin, Yijun Lv, and Zhuowu Men. 2022. "The Effect of Chlorine Modification of Precipitated Iron Catalysts on Their Fischer–Tropsch Synthesis Properties" Catalysts 12, no. 8: 812. https://doi.org/10.3390/catal12080812
APA StyleLi, W., Zhang, X., Wang, T., Zhang, X., Wei, L., Lin, Q., Lv, Y., & Men, Z. (2022). The Effect of Chlorine Modification of Precipitated Iron Catalysts on Their Fischer–Tropsch Synthesis Properties. Catalysts, 12(8), 812. https://doi.org/10.3390/catal12080812