CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterizations
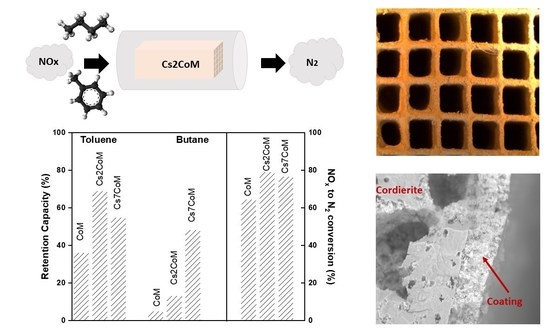
2.2. Adsorption–Desorption Study of Toluene and Butane Mixture on Monolithic Adsorbents
2.3. Selective Catalytic Reduction of NOx Using Toluene and Butane Mixture in the Presence of O2 Excess
3. Materials and Methods
3.1. Powder Preparation
3.2. Monolithic Structures Preparation
3.3. Adsorption and Temperature-Programmed Desorption of Mixture Toluene-Butane
3.4. Catalytic Evaluation on Selective Catalytic Reduction of NOx
3.5. Physicochemical Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Tian, J.; Li, J.; Cao, C.; Wang, S.; Lv, J.; Zheng, W.; Tan, D. The Development of Diesel Oxidation Catalysts and the Effect of Sulfur Dioxide on Catalysts of Metal-Based Diesel Oxidation Catalysts: A Review. Fuel Process. Technol. 2022, 233, 107317. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline Automobile Catalysis and Its Historical Journey to Cleaner Air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Casapu, M. Present Challenges in Catalytic Emission Control for Internal Combustion Engines. Catalysts 2021, 11, 1019. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle Emissions Trapping Materials: Successes, Challenges, and the Path Forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Barbera-Italiano, K.; Jeudy, E.; Lecompte, M.; Laigle, E.; Norsic, C.; Chaillou, C.; Bourhis, G. Trap Efficiency of Exhaust Gas Pollutants in Microporous Sorbents under Representative Driving Conditions. Appl. Catal. B Environ. 2022, 304, 120962. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Finqueneisel, G.; Da Costa, P.; Can, F. Evolution of Unburnt Hydrocarbons under “Cold-Start” Conditions from Adsorption/Desorption to Conversion: On the Screening of Zeolitic Materials. Appl. Catal. B Environ. 2014, 158–159, 48–59. [Google Scholar] [CrossRef]
- Zelinsky, R.P.; Dean, D.P.; Breckner, C.J.; Marino, S.; Miller, J.T.; Epling, W.S. Pd/BEA Hydrocarbon Traps: Effect of Hydrothermal Aging on Trapping Properties and Pd Speciation. Appl. Catal. B Environ. 2023, 320, 121938. [Google Scholar] [CrossRef]
- Ryu, T.; Jeong, J.; Byun, S.W.; Kweon, S.; Park, J.; Bae, W.B.; Kim, D.Y.; Kim, Y.J.; Park, M.B.; Kang, S.B. Ethylene Trapping of Palladium-Impregnated Zeolites for Cold-Start Emission Control. Chem. Eng. J. 2022, 442, 136197. [Google Scholar] [CrossRef]
- Kim, J.; Jang, E.; Jeong, Y.; Baik, H.; Cho, S.J.; Kang, C.Y.; Kim, C.H.; Choi, J. A Cu-Impregnated ZSM-5 Zeolite for Active Cold Start Hydrocarbon Removal: Cation-Type-Dependent Cu Species and Their Synergetic HC Adsorption/Oxidation Functions. Chem. Eng. J. 2022, 430, 132552. [Google Scholar] [CrossRef]
- Lee, J.; Giewont, K.; Chen, J.; Liu, C.-H.; Walker, E.A.; Kyriakidou, E.A. Ag/ZSM-5 Traps for C2H4 and C7H8 Adsorption under Cold-Start Conditions. Microporous Mesoporous Mater. 2021, 327, 111428. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Chebbi, M.; Koch, A. Modification of Y Faujasite Zeolites for the Trapping and Elimination of a Propene-Toluene-Decane Mixture in the Context of Cold-Start. Microporous Mesoporous Mater. 2016, 230, 76–88. [Google Scholar] [CrossRef]
- Azambre, B.; Westermann, A.; Finqueneisel, G.; Can, F.; Comparot, J.D. Adsorption and Desorption of a Model Hydrocarbon Mixture over HY Zeolite under Dry and Wet Conditions. J. Phys. Chem. C 2015, 119, 315–331. [Google Scholar] [CrossRef]
- Serra, R.M.; Miró, E.E.; Boix, A.V. FTIR Study of Toluene Adsorption on Cs-Exchanged Mordenites. Microporous Mesoporous Mater. 2010, 127, 182–189. [Google Scholar] [CrossRef]
- Serra, R.M.; Miró, E.E.; Sapag, M.K.; Boix, A.V. Adsorption and Diffusion of Toluene on Na and Cs Mordenites for Hydrocarbon Traps. Microporous Mesoporous Mater. 2011, 138, 102–109. [Google Scholar] [CrossRef]
- Serra, R.M.; Miró, E.E.; Bolcatto, P.; Boix, A.V. Experimental and Theoretical Studies about the Adsorption of Toluene on ZSM5 and Mordenite Zeolites Modified with Cs. Microporous Mesoporous Mater. 2012, 147, 17–29. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yahiro, H. Novel Catalytic Decomposition and Reduction of NO. Catal. Today 1994, 22, 5–18. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx Selective Catalytic Reduction by Hydrocarbons (HC-SCR). Appl. Catal. A Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Charrad, R.; Solt, H.E.; Domján, A.; Ayari, F.; Mhamdi, M.; Valyon, J.; Lónyi, F. Selective Catalytic Reduction of NO by Methane over Co,H-SSZ-13 Catalysts: Types and Catalytic Functions of Active Co Sites. J. Catal. 2020, 385, 87–102. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Miró, E.E.; Boix, A.V. Effect of Ag–Co Interactions in the Mordenite on the NOx SCR with Butane and Toluene. Catal. Commun. 2012, 28, 105–110. [Google Scholar] [CrossRef]
- Rokicińska, A.; Drozdek, M.; Bogdan, E.; Węgrzynowicz, A.; Michorczyk, P.; Kuśtrowski, P. Combustion of Toluene over Cobalt-Modified MFI Zeolite Dispersed on Monolith Produced Using 3D Printing Technique. Catal. Today 2021, 375, 369–376. [Google Scholar] [CrossRef]
- Boix, A.V.; Zamaro, J.M.; Lombardo, E.A.; Miró, E.E. The Beneficial Effect of Silica on the Activity and Thermal Stability of PtCoFerrierite-Washcoated Cordierite Monoliths for the SCR of NOx with CH4. Appl. Catal. B Environ. 2003, 46, 121–132. [Google Scholar] [CrossRef]
- Boix, A.V.; Miró, E.E.; Lombardo, E.A.; Mariscal, R.; Fierro, J.L.G. Binder Effect upon the Catalytic Behavior of PtCoZSM5 Washcoated on Cordierite Monoliths. Appl. Catal. A Gen. 2004, 276, 197–205. [Google Scholar] [CrossRef]
- Boix, A.V.; Lombardo, E.A.; Miró, E.E. Effect of the Pt/Co Ratio upon the Catalytic Behavior of PtCoFerrierite Washcoated on Cordierite Monoliths. Catal. Today 2005, 107–108, 330–337. [Google Scholar] [CrossRef]
- Boix, A.V.; Aspromonte, S.G.; Miró, E.E. Deactivation Studies of the SCR of NOx with Hydrocarbons on Co-Mordenite Monolithic Catalysts. Appl. Catal. A Gen. 2008, 341, 26–34. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. Advance in the Scaling up of a Hybrid Catalyst for NSR-SCR Coupled Systems under H2O + CO2 Atmosphere. Catal. Today 2020, 356, 292–300. [Google Scholar] [CrossRef]
- Azzoni, M.E.; Franchi, F.S.; Usberti, N.; Nasello, N.D.; Castoldi, L.; Nova, I.; Tronconi, E. Dual-Layer AdSCR Monolith Catalysts: A New Solution for NOx Emissions Control in Cold Start Applications. Appl. Catal. B Environ. 2022, 315, 121544. [Google Scholar] [CrossRef]
- Serra, R.M.; Aspromonte, S.G.; Miró, E.E.; Boix, A.V. Hydrocarbon Adsorption and NOx-SCR on (Cs,Co)Mordenite. Appl. Catal. B Environ. 2015, 166–167, 592–602. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Pászti, Z.; Valyon, J. Mechanism of NO-SCR by Methane over Co,H-ZSM-5 and Co,H-Mordenite Catalysts. Appl. Catal. B Environ. 2014, 150–151, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Boix, A.; Fierro, J.L.G. X-Ray Photoelectron Spectroscopy Analysis of Platinum- and/or Cobalt-Loaded Zeolites Relevant for Selective Catalytic Reduction of NOx. Surf. Interface Anal. 1999, 27, 1107–1113. [Google Scholar] [CrossRef]
- Zsoldos, Z.; Vass, G.; Lu, G.; Guczi, L. XPS Study on the Effects of Treatments on Pt2+ and Co2+ Exchanged into NaY Zeolite. Appl. Surf. Sci. 1994, 78, 467–475. [Google Scholar] [CrossRef]
- Dutta, N.C.; Iwasaki, T.; Ebina, T.; Hayashi, H. A Combined X-Ray Photoelectron and Auger Electron Spectroscopic Study of Cesium in Variable-Charge Montmorillonites. J. Colloid Interface Sci. 1999, 216, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Wang, C.B.; Chien, S.H. Characterization of Cobalt Oxides Studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Gómez, L.E.; Múnera, J.F.; Sollier, B.M.; Miró, E.E.; Boix, A.V. Raman in Situ Characterization of the Species Present in Co/CeO2 and Co/ZrO2 Catalysts during the COPrOx Reaction. Int. J. Hydrogen Energy 2016, 41, 4993–5002. [Google Scholar] [CrossRef]
- Boix, A.; Miró, E.E.; Lombardo, E.A.; Bañares, M.A.; Mariscal, R.; Fierro, J.L.G. The Nature of Cobalt Species in Co and PtCoZSM5 Used for the SCR of NOx with CH4. J. Catal. 2003, 217, 186–194. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. A Study on the Roles of Cobalt Species in NOx Reduction by Propane on Co-Beta. Catal. Today 1998, 42, 45–50. [Google Scholar] [CrossRef]
- Band, A.; Albu-Yaron, A.; Livneh, T.; Cohen, H.; Feldman, Y.; Shimon, L.; Popovitz-Biro, R.; Lyahovitskaya, V.; Tenne, R. Characterization of Oxides of Cesium. J. Phys. Chem. B 2004, 108, 12360–12367. [Google Scholar] [CrossRef]
- Kraus, M.; Trommler, U.; Holzer, F.; Kopinke, F.-D.; Roland, U. Competing Adsorption of Toluene and Water on Various Zeolites. Chem. Eng. J. 2018, 351, 356–363. [Google Scholar] [CrossRef]
- Hussein, M.S.; Ahmed, M.J. Fixed Bed and Batch Adsorption of Benzene and Toluene from Aromatic Hydrocarbons on 5A Molecular Sieve Zeolite. Mater. Chem. Phys. 2016, 181, 512–517. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of Acid Activation of Palygorskite on Their Toluene Adsorption Behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Kustov, L.; Golubeva, V.; Korableva, A.; Anischenko, O.; Yegorushina, N.; Kapustin, G. Alkaline-Modified ZSM-5 Zeolite to Control Hydrocarbon Cold-Start Emission. Microporous Mesoporous Mater. 2018, 260, 54–58. [Google Scholar] [CrossRef]
- Takamitsu, Y.; Ariga, K.; Yoshida, S.; Ogawa, H.; Sano, T. Adsorption of Toluene on Alkali Metal Ion-Exchanged ZSM-5 and β-Zeolites under Humid Conditions. Bull. Chem. Soc. Jpn. 2012, 85, 869–876. [Google Scholar] [CrossRef]
- William Corning Inc. Modified Large Pore Zeolites for Trapping Alkenes. Patent EP 0639400 A1, 4 April 2016. [Google Scholar]
- Martins, L.; Holderich, W.; Cardoso, D. Methylammonium-FAU Zeolite: Investigation of the Basic Sites in Base Catalyzed Reactions and Its Performance. J. Catal. 2008, 258, 14–24. [Google Scholar] [CrossRef]
- Oh, H.; Beum, H.T.; Yoon, Y.-S.; Kim, J.; Han, Y.; Kim, J.; Lee, I.-B.; Lee, S.-Y.; Han, S.S. Experiment and Modeling of Adsorption of CO from Blast Furnace Gas onto CuCl/Boehmite. Ind. Eng. Chem. Res. 2020, 59, 12176–12185. [Google Scholar] [CrossRef]
- Czaplewski, K.F.; Reitz, T.L.; Kim, Y.J.; Snurr, R.Q. One-Dimensional Zeolites as Hydrocarbon Traps. Microporous Mesoporous Mater. 2002, 56, 55–64. [Google Scholar] [CrossRef]
- Wesson, P.J.; Snurr, R.Q. Modified Temperature Programmed Desorption Evaluation of Hydrocarbon Trapping by CsMOR Zeolite under Cold Start Conditions. Microporous Mesoporous Mater. 2009, 125, 35–38. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B. Impact of the Zeolite Structure and Acidity on the Adsorption of Unburnt Hydrocarbons Relevant to Cold Start Conditions. J. Phys. Chem. C 2016, 120, 25903–25914. [Google Scholar] [CrossRef]
- Zhao, W.; Ruan, S.; Qian, S.; Feng, B.; Ao, C.; Wang, L.; Liu, F.; Zhang, L. Abatement of n -Butane by Catalytic Combustion over Co-ZSM-5 Catalysts. Energy Fuels 2020, 34, 12880–12890. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Shamzhy, M.; Molitorisová, S.; Opanasenko, M.; Giroir-Fendler, A. Total Oxidation of Toluene and Propane over Supported Co3O4 Catalysts: Effect of Structure/Acidity of MWW Zeolite and Cobalt Loading. ACS Appl. Mater. Interfaces 2021, 13, 15143–15158. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Dujardin, C.; Góra-Marek, K.; Granger, P.; Sojka, Z. Spectroscopic IR, EPR, and OperandoDRIFT Insights into Surface Reaction Pathways of Selective Reduction of NO by Propene over the Co–BEAzeolite. Phys. Chem. Chem. Phys. 2012, 14, 2203–2215. [Google Scholar] [CrossRef]
- Shilina, M.I.; Rostovshchikova, T.N.; Nikolaev, S.A.; Udalova, O.V. Polynuclear Co-Oxo Cations in the Catalytic Oxidation of CO on Co-Modified ZSM-5 Zeolites. Mater. Chem. Phys. 2019, 223, 287–298. [Google Scholar] [CrossRef]
- Lim, J.B.; Shin, J.; Ahn, N.H.; Heo, I.; Hong, S.B. Selective Catalytic Reduction of NO with CH4 over Cobalt-Exchanged Cage-Based, Small-Pore Zeolites with Different Framework Structures. Appl. Catal. B Environ. 2020, 267, 118710. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Boix, A.V. The Nature of Cobalt Species in Co-Zeolites Used for the Selective Catalytic Reduction of NOx with Hydrocarbons. In Cobalt: Occurrence, Uses and Properties; Kobayashi, Y., Suzuki, H., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; p. 135. ISBN 978-1-62808-278-4. [Google Scholar]
- Wu, D.; Zhang, H. Mechanical Stability of Monolithic Catalysts: Scattering of Washcoat Adhesion and Failure Mechanism of Active Material. Ind. Eng. Chem. Res. 2013, 52, 14713–14721. [Google Scholar] [CrossRef]









| Powder Composition | Na (wt.%) | Co (wt.%) | Cs (wt.%) | Monolithic Sample |
|---|---|---|---|---|
| Na-MOR | 4.2 | --- | --- | M |
| Co/Na-MOR | 1.8 | 2.9 | --- | CoM |
| Cs2/Na-MOR | 3.2 | --- | 1.9 | Cs2M |
| Cs7/Na-MOR | 2.7 | --- | 7.1 | Cs7M |
| Cs2-Co/Na-MOR | 1.6 | 2.5 | 1.8 | Cs2CoM |
| Cs7-Co/Na-MOR | 1.1 | 2.5 | 7.3 | Cs7CoM |
| Monolithic Samples | a QBads (µmol/mg) | b QBdes (µmol/mg) | a QTads (µmol/mg) | b QTdes (µmol/mg) |
|---|---|---|---|---|
| M | 0.10 | 0.03 | 0.50 | 0.35 |
| CoM | 0.02 | 0.001 | 0.11 | 0.04 |
| Cs2M | 0.11 | 0.04 | 0.55 | 0.40 |
| Cs7M | 0.07 | 0.01 | 0.35 | 0.25 |
| Cs2CoM | 0.09 | 0.01 | 0.45 | 0.31 |
| Cs7CoM | 0.06 | 0.03 | 0.31 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, R.M.; Gómez, L.E.; Tiscornia, I.S.; Deharbe, M.d.l.M.; Boix, A.V. CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx. Catalysts 2023, 13, 106. https://doi.org/10.3390/catal13010106
Serra RM, Gómez LE, Tiscornia IS, Deharbe MdlM, Boix AV. CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx. Catalysts. 2023; 13(1):106. https://doi.org/10.3390/catal13010106
Chicago/Turabian StyleSerra, Ramiro M., Leticia E. Gómez, Inés S. Tiscornia, María de los Milagros Deharbe, and Alicia V. Boix. 2023. "CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx" Catalysts 13, no. 1: 106. https://doi.org/10.3390/catal13010106
APA StyleSerra, R. M., Gómez, L. E., Tiscornia, I. S., Deharbe, M. d. l. M., & Boix, A. V. (2023). CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx. Catalysts, 13(1), 106. https://doi.org/10.3390/catal13010106