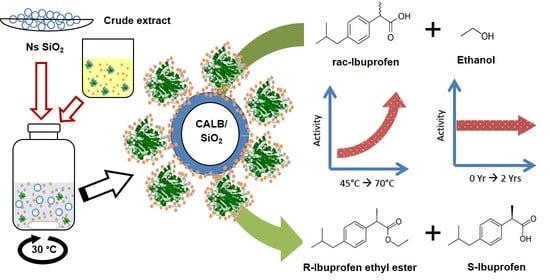
Rational Design of a Biocatalyst Based on Immobilized CALB onto Nanostructured SiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Key Features of the Immobilization of CALB on Ns Silica: Maximum Dispersion Limit of Protein and Mechanism of Adsorption
2.2. Immobilization Performance and Activity–Structure Relationship of the Adsorption Process
2.3. Kinetic Resolution of rac-Ibuprofen: Catalytic Activity—Maximum Dispersion Limit Relationship
2.4. Lipase Co-Adsorption with Polyols onto Ns SiO2: Influence on the Catalytic Performance
2.5. Influence of the Temperature on the Catalytic Performance
2.6. Stability of CALB Immobilized on Ns SiO2: Influence of the Temperature and Storage
3. Materials and Methods
3.1. Materials
3.2. Raman and Infrared (DRIFTS) Spectroscopy Analysis
3.3. Immobilization of CALB onto SiO2: Kinetic of Adsorption
3.4. Isotherm of Adsorption: Maximum Dispersion Limit
3.5. SDS-PAGE Analysis
3.6. Hydrolysis of p-Nitrophenyl Dodecanoate
3.7. Enantioselective Esterification of rac-Ibuprofen
3.8. Stability under Thermal Stress and after Extended Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemli, S.; Ayadi-Zouari, D.; Hlima, H.B.; Bejar, S. Biocatalysts: Applications and engineering for industrial purposes. Crit. Rev. Biotechnol. 2016, 32, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Bayer, T.; Badenhorst, C.P.S.; Wu, S.; Doerr, M.; Höhne, M.; Bornscheuer, U.T. Recent trends in biotechnology. Chem. Soc. Rev. 2021, 50, 8003–8049. [Google Scholar] [PubMed]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilization and stabilization with potential applications in biodiesel production. Appl. MicroBiol. Biot. 2013, 97, 23–39. [Google Scholar] [CrossRef]
- Wang, P. Multi-scale features in recent development of enzymatic biocatalyst systems. Appl. BioChem. Biotech. 2009, 152, 343–352. [Google Scholar] [CrossRef]
- Shuai, W.; Das, R.K.; Naghdi, M.; Brar, S.K.; Verma, M. A review on the important aspects of lipase immobilization on nanomaterials. Biotechnol. Appl. BioChem. 2017, 64, 496–508. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nanomaterials: A review. Biotech. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar]
- Zhao, L.; Zhang, Y.; Yang, Y.; Yu, C. Silica-based nanoparticles for enzyme immobilization and delivery. Chem. Asian J. 2022, 17, e202200573. [Google Scholar] [CrossRef]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. WIRes. Nanomed. Nanobiotechnol. 2021, 13, e1658. [Google Scholar]
- Sengupta, S.; Das, P.; Sharma, S.; Shukla, M.K.; Kumar, R.; Tonk, R.K.; Pandey, S.; Kumar, D. Role and application of biocatalysts in cancer drug discovery. Catalysts 2023, 13, 250. [Google Scholar]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.C.S.; dos Santos, J.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar]
- Cruz, J.C.; Pfromm, P.H.; Rezac, M.E. Immobilization of Candida antarctica Lipase B on fumed silica. Process BioChem. 2009, 44, 62–69. [Google Scholar]
- Serra, E.; Díez, E.; Díaz, I.; Blanco, R.M. A comparative study of periodic mesoporous organosilica and different hydrophobic mesoporous silicas for lipase immobilization. Microporous Microporous Mater. 2010, 132, 487–493. [Google Scholar]
- Cassimjee, K.E.; Kourist, R.; Lindberg, D.; Wittrup, L.M.; Thanh, N.H.; Widersten, M.; Berglund, P. One-step enzyme extraction and immobilization for biocatalysis applications. Biotechnol. J. 2011, 6, 463–469. [Google Scholar]
- Gandomkar, S.; Habibi, Z.; Mohammadi, M.; Yousefi, M.; Salimi, S. Enantioselective resolution of racemic ibuprofen using different lipases immobilized on epoxy-functionalized silica. Biocatal. Agric. Biotechnol. 2015, 4, 550–554. [Google Scholar]
- Mittersteiner, M.; Linshalm, B.L.; Vieira, A.P.F.; Brondani, P.B.; Scharf, D.R.; de Jesus, P.C. Convenient enzymatic resolution of (R, S)-2-methylbutyric acid catalyzed by immobilized lipases. Chirality 2018, 30, 106–111. [Google Scholar]
- Vesoloski, J.; Todero, A.; Macieski, R.; de Oliveira Pereira, F.; Dallago, R.; Mignoni, M. Immobilization of lipase from Candida antarctica B (CALB) by sol–gel technique using rice Husk ash as silic source and ionic liquid as additive. Appl. BioChem. Biotechnol. 2022, 194, 6270–6286. [Google Scholar]
- José, C.; Toledo, M.V.; Briand, L.E. Enzymatic kinetic resolution of ibuprofen: Past, present and future. Crit. Rev. Biotechnol. 2016, 36, 891–903. [Google Scholar]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- José, C.; Toledo, M.V.; Grisales, J.O.; Briand, L.E. Effect of co-solvents in the enantioselective esterification of (R/S)-ibuprofen with ethanol. Curr. Catal. 2014, 3, 131–138. [Google Scholar] [CrossRef]
- Llerena Suster, C.R.; Toledo, M.V.; Fittipaldi, A.S.; Morcelle, S.R.; Briand, L.E. Lipase B of Candida antarctica co-adsorbed with polyols onto TiO2 nanoparticles for improved biocatalytic performance. J. Chem. Technol. Biotechnol. 2017, 92, 2870–2880. [Google Scholar] [CrossRef]
- Toledo, M.V.; José, C.; Llerena Suster, C.R.; Collins, S.E.; Portela, R.; Bañares, M.A.; Briand, L.E. Catalytic and molecular insights of the esterification of ibuprofen and ketoprofen with glycerol. Mol. Catal. 2021, 513, 111811–111819. [Google Scholar]
- Lee, E.L.; Wachs, I.E. In situ Raman spectroscopy of SiO2-supported transition metal oxide catalysts: An isotopic 18O-16O exchange study. J. Phys. Chem. C 2008, 112, 6487–6498. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Llerena Suster, C.R.; Briand, L.E.; Morcelle, S.R. Analytical characterization and purification of a commercial extract of enzymes: A case study. Colloid Surf. B 2014, 121, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. pH-dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Barisik, M.; Atalay, S.; Beskok, A.; Qian, S. Size dependent surface charge properties of silica nanoparticles. J. Phys. Chem. C 2014, 118, 1836–1842. [Google Scholar] [CrossRef]
- Meissner, J.; Prause, A.; Bharti, B.; Findenegg, G.H. Characterization of protein adsorption on silica nanoparticles: Influence of pH and ionic strength. Colloid Polym. Sci. 2015, 293, 3381–3391. [Google Scholar] [CrossRef] [Green Version]
- Neves Petersen, M.T.; Fojan, P.; Pettersen, S.B. How do lipases and esterases work: The electrostatic contribution. J. Biotechnol. 2001, 85, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.L.; Najafpour, G.D.; Moghadamnia, A.; Kamaruddin, A.H. Kinetics and isotherm studies of the immobilized lipase on chitosan support. IJE Trans. 2016, 29, 1319–1331. [Google Scholar]
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, M.V.; José, C.; Collins, S.E.; Ferreira, M.L.; Briand, L.E. Towards a green enantiomeric esterification of R/S-ketoprofen: A theoretical and experimental investigation. J. Mol. Catal. B Enzym. 2015, 118, 52–61. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Improved thermal stability and activity in the cold-adapted lipase B from Candida antarctica following chemical modification with oxidized polysaccharides. Extremophiles 2005, 9, 471–476. [Google Scholar] [CrossRef]
- Arroyo, M.; Sánchez-Montero, J.M.; Sinisterra, J.V. Thermal stabilization of immobilized lipase B from Candida antarctica on different supports: Effect of water activity on enzymatic activity in organic media. Enzym. Microb. Technol. 1999, 24, 3–12. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Battiston, C.S.Z.; Ficanha, A.M.M.; Levandoski, K.L.D.; da Silva, B.A.; Battiston, S.; Dallago, R.M.; Mignoni, M.L. Immobilization of lipase on mesoporous molecular sieve MCM-48 obtained using ionic solid as a structure director and esterification reaction on solvent-free. Quim. Nova 2017, 40, 293–298. [Google Scholar] [CrossRef]
- Report of Storage Stability of Novozym®435 from Novozyme Co. Published on 30 January 30 2018. Available online: http://www.cliscent.com (accessed on 5 March 2023).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. BioChem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Alam, M.; Islam, N. Study on water sorption isotherm of summer onion. Bangladesh J. Agric. Res. 2015, 40, 35–51. [Google Scholar]
- Adeogun, A.I.; Kareeem, S.O.; Adebayo, O.S.; Balogun, S.A. Comparative adsorption of amylase, protease and lipase on ZnFe2O4: Kinetics, isothermal and thermodynamics studies. 3 Biotech. 2017, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Blahovek, J.; Yanniotis, S. ‘Gab’ generalized equation as a basis for sorption spectra analysis. Czech J. Food Sci. 2010, 28, 354. [Google Scholar]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]












| Adsorption Model | Parameters | |
|---|---|---|
| Langmuir | QMAX (μmol/m2) | 0.036 ± 0.002 |
| KL (mg/mL) | 5.95 | |
| R2 | 0.91 | |
| Σ2 | 6.89 | |
| Freundlich | KF (μmol/m2) | 0.033 ± 0.002 |
| nF | 3.00 | |
| R2 | 0.87 | |
| Σ2 | 10.99 | |
| Hill | QMAX (μmol/m2) | 0.032 ± 0.007 |
| KH | 0.2 | |
| nH | 1.5 | |
| R2 | 0.89 | |
| Σ2 | 6.86 | |
| GAB | Wm (μmol/m2) | 0.033 ± 0.002 |
| C | 107.12 | |
| k | 0.06 | |
| R2 | 0.97 | |
| Σ2 | 5.65 | |
| Dubinin-Radushkevich | Xm (μmol/m2) | 0.028 ± 0.009 |
| β | −2.22 × 10−8 | |
| E (kJ/mol) | −4.74 | |
| R2 | 0.94 | |
| Σ2 | 0.02 | |
| Yield % | |||||
|---|---|---|---|---|---|
| Protein Solution | Protein Concentration (mg.mL−1) | Activity (µmol.min−1.mL−1) | Immobilized Protein | Residual Activity | |
| Assay I | Starting | 1.91 ± 0.09 | 0.177 ± 0.004 | 61.7 | 79.7 |
| equilibrium | 0.73 ± 0.03 | 0.036 ± 0.005 | |||
| Assay II | Starting | 1.60 ± 0.07 | 0.118 ± 0.005 | 62.5 | 69.5 |
| equilibrium | 0.6 ± 0.1 | 0.036 ± 0.003 | |||
| Sample | Source of CALB | Glycerol–Sorbitol (% p/v) | Protein Density (µmol.m−2) | Specific Activity (µmol.min−1.mg−1) |
|---|---|---|---|---|
| A | pure | ------ | 0.021 ± 0.002 | 0.006 ± 0.001 |
| B * | Crude extract | 3% a | 0.017 ± 0.002 | 0.219 ± 0.006 |
| B | Crude extract | 3% a | 0.019 ± 0.002 | 0.194 ± 0.010 |
| C | Crude extract | 6% b | 0.018 ± 0.002 | 0.189 ± 0.012 |
| D | Crude extract | 9% c | 0.023 ± 0.001 | 0.232 ± 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llerena Suster, C.R.; Toledo, M.V.; Matkovic, S.R.; Morcelle, S.R.; Briand, L.E. Rational Design of a Biocatalyst Based on Immobilized CALB onto Nanostructured SiO2. Catalysts 2023, 13, 625. https://doi.org/10.3390/catal13030625
Llerena Suster CR, Toledo MV, Matkovic SR, Morcelle SR, Briand LE. Rational Design of a Biocatalyst Based on Immobilized CALB onto Nanostructured SiO2. Catalysts. 2023; 13(3):625. https://doi.org/10.3390/catal13030625
Chicago/Turabian StyleLlerena Suster, Carlos R., María V. Toledo, Silvana R. Matkovic, Susana R. Morcelle, and Laura E. Briand. 2023. "Rational Design of a Biocatalyst Based on Immobilized CALB onto Nanostructured SiO2" Catalysts 13, no. 3: 625. https://doi.org/10.3390/catal13030625
APA StyleLlerena Suster, C. R., Toledo, M. V., Matkovic, S. R., Morcelle, S. R., & Briand, L. E. (2023). Rational Design of a Biocatalyst Based on Immobilized CALB onto Nanostructured SiO2. Catalysts, 13(3), 625. https://doi.org/10.3390/catal13030625