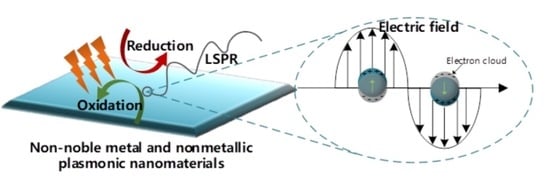
Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications
Abstract
:1. Introduction
2. Non-Noble Metal Plasmonic Nanomaterials
2.1. Bi-Based Composite Photocatalysts
2.2. Cu-Based Photocatalysts
2.3. Al-Based Photocatalysts
2.4. Ni-Based Photocatalysts
3. Nonmetallic Plasmonic Nanomaterials
3.1. Extrinsic Doping Metal Oxides/Semiconductors
3.2. Self-Doped Metal Oxides/Semiconductors
4. Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Yang, W.; Li, Q. TiO2-based photocatalysts prepared by oxidation of TiN nanoparticles and their photocatalytic activities under visible light illumination. J. Mater. Sci. Technol. 2018, 34, 969–975. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. Ternary Pt@TiO2/rGO Nanocomposite to Boost Photocatalytic Activity for Environmental and Energy Use. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3802–3809. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ahn, H.-J.; Youn, J.-I.; Kim, Y.-J.; Suh, S.-J.; Oh, H.-J. Synthesis and Characterization of ZnO/TiO2 Photocatalyst Decorated with PbS QDs for the Degradation of Aniline Blue Solution. Korean J. Met. Mater. 2018, 56, 900–909. [Google Scholar] [CrossRef]
- Sheikhsamany, R.; Faghihian, H. Elimination of Phenylhydrazine from Aqueous Solutions by Use of a Photocatalyst Prepared by Immobilization of TiO2 on Polypyrrole Support. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1980–1989. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Wang, P.; Cheng, H.; Zheng, Z.; Lou, Z.; Dai, Y. Plasmonic Photocatalysts with Wide Light Absorption Spectra and High Charge Separation Efficiencies. In From Molecules to Materials: Pathways to Artificial Photosynthesis; Rozhkova, E.A., Ariga, K., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–267. [Google Scholar]
- Li, Q.; Lei, S.; Li, Y.; Wang, Y.; Zhao, B.; Ruan, W. Investigation of compositionally tunable localized surface plasmon resonances (LSPRs) of a series of indium tin oxide nanocrystals prepared by one-step solvothermal synthesis. J. Mater. Sci. 2018, 54, 2918–2927. [Google Scholar] [CrossRef]
- Chang, H.; Rho, W.-Y.; Son, B.S.; Kim, J.; Lee, S.H.; Jeong, D.H.; Jun, B.-H. Plasmonic Nanoparticles: Basics to Applications (I). In Nanotechnology for Bioapplications; Jun, B.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1309, pp. 133–159. [Google Scholar]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. In situ ion exchange synthesis of strongly coupled Ag@AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 22116–22125. [Google Scholar] [CrossRef]
- Garcia-Peiro, J.I.; Bonet-Aleta, J.; Bueno-Alejo, C.J.; Hueso, J.L. Recent Advances in the Design and Photocatalytic Enhanced Performance of Gold Plasmonic Nanostructures Decorated with Non-Titania Based Semiconductor Hetero-Nanoarchitectures. Catalysts 2020, 10, 1459. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Li, H.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.J.P.; Characterization, P.S. One-step hydrothermal synthesis of Ag nanoparticle decorated submicrometer-scale spherical AgBr colloids: A highly efficient visible light plasmonic photocatalyst for degradation of organic dyes. Part. Part. Syst. Charact. 2013, 30, 67–71. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Liu, L.; Li, H.; Xu, Y.; Xia, J.; Wu, X.; Zhao, S. Plasmonic-enhanced visible-light-driven photocatalytic activity of Ag–AgBr synthesized in reactable ionic liquid. J. Chem. Technol. Biotechnol. 2012, 87, 1626–1633. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, S.; Liu, L.; Hu, J.; Cui, W. Facile hydrothermal synthesis of plasmonic photocatalyst Ag@AgCl and degradative photocatalysis under visible light irradiation. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2015, 30, 84–91. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G.; Mi, J.; Wu, Z. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, Z.F.; Jing, J.J.; Lv, X.M.; Xie, J.M. Smart strategy to synthesis silver-based heterogeneous photocatalysts grown from molybdenum oxide precursor. Micro Nano Lett. 2017, 12, 978–981. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An Inorganic/Organic Hybrid Plasmonic Photocatalyst with Enhanced Hydrogen Gas Evolution Under Visible-Light Irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar]
- Chang, A.; Peng, W.-S.; Tsai, I.T.; Chiang, L.-F.; Yang, C.-M. Efficient hydrogen production by selective alcohol photoreforming on plasmonic photocatalyst comprising sandwiched Au nanodisks and TiO2. Appl. Catal. B: Environ. 2019, 255, 117773. [Google Scholar] [CrossRef]
- Cui, M.; Yu, J.; Lin, H.; Wu, Y.; Zhao, L.; He, Y. In-situ preparation of Z-scheme AgI/Bi5O7I hybrid and its excellent photocatalytic activity. Appl. Surf. Sci. 2016, 387, 912–920. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Huang, D.-W.; Zeng, G.-M. In-situ synthesis of visible-light-driven plasmonic Ag/AgCl-CdWO4 photocatalyst. Ceram. Int. 2017, 43, 1922–1929. [Google Scholar] [CrossRef]
- Song, J.; Shi, Y.; Ren, M.; Hu, G. Synthesis, characterization and excellent photocatalytic activity of Ag/AgBr/MoO3 composite photocatalyst. Appl. Phys. A 2014, 116, 2139–2147. [Google Scholar] [CrossRef]
- Song, S.; Cheng, B.; Wu, N.; Meng, A.; Cao, S.; Yu, J. Structure effect of graphene on the photocatalytic performance of plasmonic Ag/Ag2CO3-rGO for photocatalytic elimination of pollutants. Appl. Catal. B: Environ. 2016, 181, 71–78. [Google Scholar] [CrossRef]
- Ren, H.; Yang, J.-L.; Yang, W.-M.; Zhong, H.-L.; Lin, J.-S.; Radjenovic, P.M.; Sun, L.; Zhang, H.; Xu, J.; Tian, Z.-Q.; et al. Core–Shell–Satellite Plasmonic Photocatalyst for Broad-Spectrum Photocatalytic Water Splitting. ACS Mater. Lett. 2020, 3, 69–76. [Google Scholar] [CrossRef]
- Wei, Z.; Benlin, D.; Fengxia, Z.; Xinyue, T.; Jiming, X.; Lili, Z.; Shiyin, L.; Leung, D.Y.C.; Sun, C. A novel 3D plasmonic p-n heterojunction photocatalyst: Ag nanoparticles on flower-like p-Ag2S/n-BiVO4 and its excellent photocatalytic reduction and oxidation activities. Appl. Catal. B: Environ. 2018, 229, 171–180. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Zhu, F.; Mu, F.; Zhang, L.; Dai, B.; Xu, J.; Zhu, A.; Sun, C.; Leung, D.Y.C. Study the photocatalytic mechanism of the novel Ag/p-Ag2O/n-BiVO4 plasmonic photocatalyst for the simultaneous removal of BPA and chromium(VI). Chem. Eng. J. 2019, 361, 1352–1362. [Google Scholar] [CrossRef]
- Shoueir, K.; Kandil, S.; El-hosainy, H.; El-Kemary, M. Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J. Clean. Prod. 2019, 230, 383–393. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Gao, Y.; Huang, H.; Tong, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: Insight into the role of oxygen vacancy defects and M=Ag, Au nanoparticles. Appl. Catal. B: Environ. 2020, 260, 118151. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Kowalska, E. Plasmonic Photocatalysts for Microbiological Applications. Catalysts 2020, 10, 824. [Google Scholar] [CrossRef]
- Khanam, S.; Rout, S.K. A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal-Semiconductor Heterostructures: A Review. Chemistry 2022, 4, 454–479. [Google Scholar] [CrossRef]
- Ly, N.H.; Vasseghian, Y.; Joo, S.-W. Plasmonic photocatalysts for enhanced solar hydrogen production: A comprehensive review. Fuel 2023, 344, 128087. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhao, Y.; Zhao, J.; Bouasavanh, S. SiO2-stabilized Bi nanoparticles: A high active and stable visible light photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 112–120. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Cui, W.; Sun, Y.; Jiang, G.; Zhang, Y.; Huang, H.; Dong, F. Bismuth spheres assembled on graphene oxide: Directional charge transfer enhances plasmonic photocatalysis and in situ DRIFTS studies. Appl. Catal. B Environ. 2018, 221, 482–489. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, R.; Lu, H.; Xie, C.a.; Ao, W.; Chen, D.; Ma, C.; Yao, W.; Zhu, Y. One-pot synthesis of C/Bi/Bi2O3 composite with enhanced photocatalytic activity. Appl. Catal. B Environ. 2017, 219, 63–72. [Google Scholar] [CrossRef]
- Zhang, L.; Ghimire, P.; Phuriragpitikhon, J.; Jiang, B.; Goncalves, A.A.S.; Jaroniec, M. Facile formation of metallic bismuth/bismuth oxide heterojunction on porous carbon with enhanced photocatalytic activity. J. Colloid Interface Sci. 2018, 513, 82–91. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, S.; Yang, Y.; Qin, H.; Ni, Z.; Yang, S.; Li, X. Facile synthesis of Bi/Bi2WO6 nanocomposite with enhanced photocatalytic activity under visible light. Appl. Catal. B Environ. 2016, 196, 89–99. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Liu, Y.; Zhou, Y.; Deng, Y.; Wang, J.; Peng, B. Plasmonic Bi Metal Deposition and g-C3N4 Coating on Bi2WO6 Microspheres for Efficient Visible-Light Photocatalysis. ACS Sustain. Chem. Eng. 2016, 5, 1062–1072. [Google Scholar] [CrossRef]
- Liang, C.; Niu, C.G.; Zhang, L.; Wen, X.J.; Yang, S.F.; Guo, H.; Zeng, G.M. Construction of 2D heterojunction system with enhanced photocatalytic performance: Plasmonic Bi and reduced graphene oxide co-modified Bi5O7I with high-speed charge transfer channels. J. Hazard. Mater. 2019, 361, 245–258. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Tu, W.; Sheng, C.; Deng, Y.; Chen, F.; Chew, J.W. Plasmonic Bi nanoparticles and BiOCl sheets as cocatalyst deposited on perovskite-type ZnSn(OH)6 microparticle with facet-oriented polyhedron for improved visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 209, 543–553. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G.; Li, Y.; Zhang, X.; Zhang, Y.; Zhou, Y.; Dong, F. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B Environ. 2018, 239, 619–627. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Li, X.; Li, J.; Cen, W.; Li, Q.; Dong, F. Highly enhanced visible light photocatalysis and in situ FT-IR studies on Bi metal@defective BiOCl hierarchical microspheres. Appl. Catal. B Environ. 2018, 225, 218–227. [Google Scholar] [CrossRef]
- Chen, D.; Fang, J.; Lu, S.; Zhou, G.; Feng, W.; Yang, F.; Chen, Y.; Fang, Z. Fabrication of Bi modified Bi2S3 pillared g-C3N4 photocatalyst and its efficient photocatalytic reduction and oxidation performances. Appl. Surf. Sci. 2017, 426, 427–436. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhang, W.; Gao, C.; Zhang, Y.; Dong, F. Plasmonic Bi metal as cocatalyst and photocatalyst: The case of Bi/(BiO)2CO3 and Bi particles. J. Colloid Interface Sci. 2017, 485, 1–10. [Google Scholar] [CrossRef]
- Sun, D.; Huang, L.; Li, L.; Yu, Y.; Du, G.; Xu, B. Plasma enhanced Bi/Bi2O2CO3 heterojunction photocatalyst via a novel in-situ method. J. Colloid Interface Sci. 2020, 571, 80–89. [Google Scholar] [CrossRef]
- Shen, X.; Yang, Y.; Song, B.; Chen, F.; Xue, Q.; Shan, S.; Li, S. Magnetically recyclable and remarkably efficient visible-light-driven photocatalytic hexavalent chromium removal based on plasmonic biochar/bismuth/ferroferric oxide heterojunction. J. Colloid Interface Sci. 2021, 590, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Meng, L.; Dai, Y.; Zhang, M.; Sun, C.; Yang, S.; He, H.; Wang, S.; Li, H. Bi spheres SPR-coupled Cu2O/Bi2MoO6 with hollow spheres forming Z-scheme Cu2O/Bi/Bi2MoO6 heterostructure for simultaneous photocatalytic decontamination of sulfadiazine and Ni(II). J. Hazard Mater. 2020, 381, 120953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, X.; Long, H.; Rao, Y. A short review on recent progress of Bi/semiconductor photocatalysts: The role of Bi metal. Chin. Chem. Lett. 2020, 31, 2583–2590. [Google Scholar] [CrossRef]
- Gu, W.; Teng, F. SPR-promoted visible-light photocatalytic activity of Bi/ZIF hybrids. J. Photochem. Photobiol. A Chem. 2020, 400, 112679. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.; Kang, M. Bismuth quantum dots anchored one-dimensional CdS as plasmonic photocatalyst for pharmaceutical tetracycline hydrochloride pollutant degradation. Chemosphere 2022, 300, 134570. [Google Scholar] [CrossRef]
- Ma, Z.-P.; Zhang, L.; Ma, X.; Shi, F.-N. Z-scheme g-C3N4/Bi/Bi3.64Mo0.36O6.55 photocatalyst with dual charge transfer channels: Photodegradation of pollutants and mechanism insights. Sep. Purif. Technol. 2022, 297, 121435. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Xi, X.; Shen, Y.; Wang, J.; Liu, Y.; Nie, Z. Bi/Bi2O3/WO3 composite: A bifunctional plasmonic heterostructure for detection and degradation pollutions in wastewater. J. Environ. Chem. Eng. 2022, 10, 107643. [Google Scholar] [CrossRef]
- Huang, T.; Xu, Z.; Zeng, G.; Zhang, P.; Song, T.; Wang, Y.; Wang, T.; Huang, S.; Wang, T.; Zeng, H. Selective deposition of plasmonic copper on few layers graphene with specific defects for efficiently synchronous photocatalytic hydrogen production. Carbon 2019, 143, 257–267. [Google Scholar] [CrossRef]
- Zhang, P.; Zeng, G.; Song, T.; Huang, S.; Wang, T.; Zeng, H. Design of plasmonic CuCo bimetal as a nonsemiconductor photocatalyst for synchronized hydrogen evolution and storage. Appl. Catal. B Environ. 2019, 242, 389–396. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Fernández, Y.; Liu, D.; Maroto-Valer, M.; Bian, J.; Zhang, X. Photoreduction of CO2 using copper-decorated TiO2 nanorod films with localized surface plasmon behavior. Chem. Phys. Lett. 2012, 531, 149–154. [Google Scholar] [CrossRef]
- Rekeb, L.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Chainet, E. Highly broadband plasmonic Cu film modified Cu2O/TiO2 nanotube arrays for efficient photocatalytic performance. Int. J. Hydrogen Energy 2019, 44, 10541–10553. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Chen, P.; Chen, L.; Ding, F.; Tang, J.; Li, Y.-J.; Au, C.-T.; Yin, S.-F. Copper-mediated metal-organic framework as efficient photocatalyst for the partial oxidation of aromatic alcohols under visible-light irradiation: Synergism of plasmonic effect and schottky junction. Appl. Catal. B Environ. 2019, 248, 380–387. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. Plasmonic Cu nanoparticle on reduced graphene oxide nanosheet support: An efficient photocatalyst for improvement of near-infrared photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 225, 172–179. [Google Scholar] [CrossRef]
- Gellé, A.; Moores, A. Water splitting catalyzed by titanium dioxide decorated with plasmonic nanoparticles. Pure Appl. Chem. 2017, 89, 1817–1827. [Google Scholar] [CrossRef]
- Derikvandi, H.; Vosough, M.; Nezamzadeh-Ejhieh, A. A comprehensive study on the enhanced photocatlytic activity of a double-shell mesoporous plasmonic Cu(@)Cu2O/SiO2 as a visible-light driven nanophotocatalyst. Environ. Sci. Pollut. Res. Int. 2020, 27, 27582–27597. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Xiao, W.; Wang, Y.; Song, T.; Long, B.; Yin, S.; Ali, A.; Deng, G.-J. Plasmon-induced carrier separation boosts high-selective photocatalytic CO2 reduction on dagger-axe-like Cu@Co core–shell bimetal. Chem. Eng. J. 2021, 417, 129295. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, K.; Zhang, L.; Yang, Y.; Yuan, H.; Li, H.; Wang, L.; Zeng, J. Copper-Based Plasmonic Catalysis: Recent Advances and Future Perspectives. Adv. Mater. 2021, 33, e2008145. [Google Scholar] [CrossRef]
- Yao, G.-Y.; Zhao, Z.-Y. Unraveling the role of cuprous oxide and boosting solar energy conversion via interface engineering in a Cu/TiO2 plasmonic photocatalyst. J. Mater. Chem. C 2020, 8, 8567–8578. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Li, X. Plasmonic CuCo/Carbon Dots: An Unconventional Photocatalyst Used for Photocatalytic Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 17979–17987. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Li, X. Plasmon-driven engineering in bimetallic CuCo combined with reduced graphene oxide for photocatalytic overall water splitting. Appl. Surf. Sci. 2021, 559, 149865. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Li, X. Photo-reduction synthesis of Cu nanoparticles as plasmon-driven non-semiconductor photocatalyst for overall water splitting. Appl. Surf. Sci. 2021, 535, 147720. [Google Scholar] [CrossRef]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Dai, B.; Zhao, W.; Huang, H.; Li, S.; Yang, G.; Wu, H.; Sun, C.; Leung, D.Y.C. Constructing an ohmic junction of copper@ cuprous oxide nanocomposite with plasmonic enhancement for photocatalysis. J. Colloid Interface Sci. 2022, 616, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, C.; Huang, H.; Li, W.; Du, D.; Han, D.; Qiu, T.; Chu, P.K. Aluminum plasmonic photocatalysis. Sci. Rep. 2015, 5, 15288. [Google Scholar] [CrossRef]
- Robatjazi, H.; Lou, M.; Clark, B.D.; Jacobson, C.R.; Swearer, D.F.; Nordlander, P.; Halas, N.J. Site-Selective Nanoreactor Deposition on Photocatalytic Al Nanocubes. Nano Lett. 2020, 20, 4550–4557. [Google Scholar] [CrossRef]
- Wang, C.; Yang, W.-C.D.; Raciti, D.; Bruma, A.; Marx, R.; Agrawal, A.; Sharma, R. Endothermic reaction at room temperature enabled by deep-ultraviolet plasmons. Nat. Mater. 2020, 20, 346–352. [Google Scholar] [CrossRef]
- Yuan, L.; Lou, M.; Clark, B.D.; Lou, M.; Zhou, L.; Tian, S.; Jacobson, C.R.; Nordlander, P.; Halas, N.J. Morphology-Dependent Reactivity of a Plasmonic Photocatalyst. ACS Nano 2020, 14, 12054–12063. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Zhou, L.; Schlather, A.E.; Dong, L.; McClain, M.J.; Swearer, D.F.; Nordlander, P.; Halas, N.J. Al-Pd Nanodisk Heterodimers as Antenna-Reactor Photocatalysts. Nano Lett. 2016, 16, 6677–6682. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; McClain, M.J.; Manjavacas, A.; Krauter, C.M.; Tian, S.; Berg, F.; Everitt, H.O.; Carter, E.A.; Nordlander, P.; et al. Aluminum Nanocrystals as a Plasmonic Photocatalyst for Hydrogen Dissociation. Nano Lett. 2016, 16, 1478–1484. [Google Scholar] [CrossRef]
- Jiang, Q.; Ji, C.; Riley, D.J.; Xie, F. Boosting the efficiency of photoelectrolysis by the addition of non-noble plasmonic metals: Al & Cu. Nanomaterials 2018, 9, 1. [Google Scholar]
- Liu, J.; Xu, M.; Zhang, T.; Chu, X.; Shi, K.; Li, J. Al/TiO2 composite as a photocatalyst for the degradation of organic pollutants. Environ. Sci. Pollut. Res. Int. 2023, 30, 9738–9748. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Chilkalwar, A.A.; Kotkondawar, A.V.; Manwar, N.R.; Antony, P.S.; Hippargi, G.; Labhsetwar, N.; Trachtenberg, M.C.; Rayalu, S.S. Plasmonic nanostructured Zn/ZnO composite enhances carbonic anhydrase driven photocatalytic hydrogen generation. J. CO2 Util. 2017, 17, 207–212. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Li, X.; Li, Y.; Bai, H.; Li, M.; Xi, G. Plasmonic W18O49-photosensitized TiO2 nanosheets with wide-range solar light harvesting. RSC Adv. 2017, 7, 23846–23850. [Google Scholar] [CrossRef]
- Zhuang, T.-T.; Liu, Y.; Li, Y.; Zhao, Y.; Wu, L.; Jiang, J.; Yu, S.-H. Integration of Semiconducting Sulfides for Full-Spectrum Solar Energy Absorption and Efficient Charge Separation. Angew. Chem.-Int. Ed. 2016, 55, 6396–6400. [Google Scholar] [CrossRef] [PubMed]
- Franzen, S. Surface plasmon polaritons and screened plasma absorption in indium tin oxide compared to silver and gold. J. Phys. Chem. C 2008, 112, 6027–6032. [Google Scholar] [CrossRef]
- Huang, H.; Hao, Q.; Fan, X.; Luo, Z.; Hou, X.; Yang, X.; Qiu, T.; Chu, P.K. Self-assembled bundled TiO2 nanowire arrays encapsulated with indium tin oxide for broadband absorption in plasmonic photocatalysis. Phys. Chem. Chem. Phys. 2017, 19, 27059–27064. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Hao, Q.; Du, D.; Luo, X.; Qiu, T. Exploring indium tin oxide capped titanium dioxide nanolace arrays for plasmonic photocatalysis. RSC Adv. 2016, 6, 12611–12615. [Google Scholar] [CrossRef]
- Riley, C.T.; Smalley, J.S.; Post, K.W.; Basov, D.N.; Fainman, Y.; Wang, D.; Liu, Z.; Sirbuly, D.J. High-Quality, Ultraconformal Aluminum-Doped Zinc Oxide Nanoplasmonic and Hyperbolic Metamaterials. Small 2016, 12, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Guillén, C.; Herrero, J. Structural and plasmonic characteristics of sputtered SnO2:Sb and ZnO:Al thin films as a function of their thickness. J. Mater. Sci. 2016, 51, 7276–7285. [Google Scholar] [CrossRef]
- Paria, D.; Vadakkumbatt, V.; Ravindra, P.; Avasthi, S.; Ghosh, A. Unconventional plasmonic sensitization of graphene in mid-infrared. Nanotechnology 2021, 32, 315202. [Google Scholar] [CrossRef]
- Ye, X.; Reifsnyder Hickey, D.; Fei, J.; Diroll, B.T.; Paik, T.; Chen, J.; Murray, C.B. Seeded growth of metal-doped plasmonic oxide heterodimer nanocrystals and their chemical transformation. J. Am. Chem. Soc. 2014, 136, 5106–5115. [Google Scholar] [CrossRef]
- Gordon, T.R.; Paik, T.; Klein, D.R.; Naik, G.V.; Caglayan, H.; Boltasseva, A.; Murray, C.B. Shape-dependent plasmonic response and directed self-assembly in a new semiconductor building block, indium-doped cadmium oxide (ICO). Nano Lett. 2013, 13, 2857–2863. [Google Scholar] [CrossRef]
- Wolf, A.; Hartling, T.; Hinrichs, D.; Dorfs, D. Synthesis of plasmonic Cu2−xSe@ZnS core@shell nanoparticles. Chemphyschem 2016, 17, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.C.; Veeranarayanan, S.; Mohamed, M.S.; Aburto, R.R.; Mitcham, T.; Bouchard, R.R.; Ajayan, P.M.; Sakamoto, Y.; Maekawa, T.; Kumar, D.S. Multifunctional Cu2−xTe Nanocubes Mediated Combination Therapy for Multi-Drug Resistant MDA MB 453. Sci. Rep. 2016, 6, 35961. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Liu, L.; Chen, L.; Xu, J.; Ma, J.; Fang, G.; Zhu, E.; Wu, H.; Zhao, L.; et al. Near-Infrared Plasmonic-Enhanced Solar Energy Harvest for Highly Efficient Photocatalytic Reactions. Nano Lett. 2015, 15, 6295–6301. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zou, H.Y.; Liu, Z.X.; Yang, T.; Gao, M.X.; Huang, C.Z. Efficient visible-light photocatalytic heterojunctions formed by coupling plasmonic Cu2−xSe and graphitic carbon nitride. New J. Chem. 2015, 39, 6186–6192. [Google Scholar] [CrossRef]
- Marbella, L.E.; Gan, X.Y.; Kaseman, D.C.; Millstone, J.E. Correlating Carrier Density and Emergent Plasmonic Features in Cu2−xSe Nanoparticles. Nano Lett. 2017, 17, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Lim, G.-D.; Kim, S.; Ryu, S.H.; Hwang, T.-Y.; Park, K.-R.; Choa, Y.-H. Near-infrared absorbance properties of Cu2−xS/SiO2 nanoparticles and their PDMS-based composites. J. Mater. Chem. C 2018, 6, 754–760. [Google Scholar] [CrossRef]
- Qiao, L.-N.; Wang, H.-C.; Shen, Y.; Lin, Y.-H.; Nan, C.-W. Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction. Nanomaterials 2017, 7, 19. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Xie, W.; Wang, Y.; Hu, Z.; Zhang, W.; Zhao, H. Facile strategy for synthesizing non-stoichiometric monoclinic structured tungsten trioxide (WO3−x) with plasma resonance absorption and enhanced photocatalytic activity. Nanomaterials 2018, 8, 553. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Fang, Y.; Zhang, M.; Liu, K.; Dong, B. A Nonmetal Plasmonic Z-Scheme Photocatalyst with UV- to NIR-Driven Photocatalytic Protons Reduction. Adv. Mater. 2017, 29, 1606688. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhu, M.; Yang, X.; Zhang, Y.; Whangbo, M.-H.; Li, B.; Huang, B. Continual injection of photoinduced electrons stabilizing surface plasmon resonance of non-elemental-metal plasmonic photocatalyst CdS/WO3−x for efficient hydrogen generation. Appl. Catal. B Environ. 2018, 226, 10–15. [Google Scholar] [CrossRef]
- Lu, C.; Li, J.; Chen, G.; Li, B.; Lou, Z. Self-Z-scheme plasmonic tungsten oxide nanowires for boosting ethanol dehydrogenation under UV-visible light irradiation. Nanoscale 2019, 11, 12774–12780. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, J.; Yan, J.; Li, B.; Huang, B.; Lou, Z. Surface plasmon resonance and defects on tungsten oxides synergistically boost high-selective CO2 reduction for ethylene. Appl. Mater. Today 2020, 20, 100744. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Zhang, J.; Chen, K.; Huang, C.; Chen, H.; Qiu, X. Plasmonic MoO3−x nanosheets with tunable oxygen vacancies as efficient visible light responsive photocatalyst. Appl. Surf. Sci. 2019, 490, 395–402. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Wu, S.; Chew, J.W. Construction of hole-transported MoO3−x coupled with CdS nanospheres for boosting photocatalytic performance via oxygen-defects-mediated Z-scheme charge transfer. Appl. Organomet. Chem. 2019, 33, e4780. [Google Scholar] [CrossRef]
- Etman, A.S.; Abdelhamid, H.N.; Yuan, Y.; Wang, L.; Zou, X.; Sun, J. Facile Water-Based Strategy for Synthesizing MoO3−x Nanosheets: Efficient Visible Light Photocatalysts for Dye Degradation. ACS Omega 2018, 3, 2193–2201. [Google Scholar] [CrossRef]
- Jin, L.; Zheng, X.; Liu, W.; Cao, L.; Cao, Y.; Yao, T.; Wei, S. Integration of plasmonic and amorphous effects in MoO3−x spheres for efficient photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 12022–12026. [Google Scholar] [CrossRef]
- Wen, M.; Song, S.; Liu, Q.; Yin, H.; Mori, K.; Kuwahara, Y.; Li, G.; An, T.; Yamashit, H. Manipulation of plasmon-induced hot electron transport in Pd/MoO3−x@ZIF-8: Boosting the activity of Pd-catalyzed nitroaromatic hydrogenation under visible-light irradiation. Appl. Catal. B Environ. 2021, 282, 119511. [Google Scholar] [CrossRef]
- Lv, C.; Wang, L.; Liu, X.; Zhao, L.; Lan, X.; Shi, J. An efficient inverse opal (IO)-TiO2-MoO3−x for photocatalytic H2 evolution and RhB degradation—The synergy effect of IO structure and plasmonic MoO3−x. Appl. Surf. Sci. 2020, 527, 146726. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, B.; Wen, T.; Zhang, S.; Zeng, M.; Hu, N.; Su, Y.; Yang, Z.; Yang, B. A Z-scheme photocatalyst for enhanced photocatalytic H2 evolution, constructed by growth of 2D plasmonic MoO3−x nanoplates onto 2D g-C3N4 nanosheets. J. Colloid Interface Sci. 2020, 567, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yi, W.; Li, J.; Xi, G.; Li, Y.; Yang, H.; Liu, J. Direct growth of defect-rich MoO3−x ultrathin nanobelts for efficiently catalyzed conversion of isopropyl alcohol to propylene under visible light. J. Mater. Chem. A 2016, 4, 1566–1571. [Google Scholar] [CrossRef]
- Li, J.; Ye, Y.; Ye, L.; Su, F.; Ma, Z.; Huang, J.; Xie, H.; Doronkin, D.E.; Zimina, A.; Grunwaldt, J.-D.; et al. Sunlight induced photo-thermal synergistic catalytic CO2 conversion via localized surface plasmon resonance of MoO3−x. J. Mater. Chem. A 2019, 7, 2821–2830. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, T.; Wu, T.; Jin, C.; Qiao, R.; Qian, Y.; Tong, G. Polymorphous ZnO nanostructures: Zn polar surface-guided size and shape evolution mechanism and enhanced photocatalytic activity. ChemCatChem 2017, 9, 3180–3190. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Cui, T.; Tong, G.; Wu, W. Enhanced photocatalytic properties of ZnO/reduced graphene oxide sheets (rGO) composites with controllable morphology and composition. Appl. Surf. Sci. 2017, 412, 58–68. [Google Scholar] [CrossRef]
- Blemker, M.A.; Gibbs, S.L.; Raulerson, E.K.; Milliron, D.J.; Roberts, S.T. Modulation of the Visible Absorption and Reflection Profiles of ITO Nanocrystal Thin Films by Plasmon Excitation. ACS Photonics 2020, 7, 1188–1196. [Google Scholar] [CrossRef]
- Hosseinpour, Z.; Hosseinpour, S. Facile synthesis of Er:CuS flowers and their application in the photo-catalytic activity. Mater. Sci. Semicond. Process. 2017, 72, 32–36. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Shao, Y.; Chen, Y.-L.; Liu, M.-X.; Li, H.-X.; Mao, Y.-L.; Ding, S.-J. Recent progress in constructing plasmonic metal/semiconductor hetero-nanostructures for improved photocatalysis. Catalysts 2018, 8, 634. [Google Scholar] [CrossRef]
- Ma, X.C.; Dai, Y.; Yu, L.; Huang, B.B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Li, F.-T.; Wang, S.-S.; Chai, M.-J.; Liu, R.-H.; Wang, X.-J. One-step combustion synthesis of β-Bi2O3-NiO/Ni composites and their visible light photocatalytic performance. Mater. Sci. Eng. B 2014, 186, 41–47. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, Y.; Wang, Z.; Xu, X.; He, H.; Du, W.; Hu, J.; Zhou, Y. Plasmonic Cocatalyst with Electric and Thermal Stimuli Boots Solar Hydrogen Evolution. Sol. RRL 2020, 4, 2000094. [Google Scholar] [CrossRef]
- Talebi, P.; Kistanov, A.A.; Rani, E.; Singh, H.; Pankratov, V.; Pankratova, V.; King, G.; Huttula, M.; Cao, W. Unveiling the role of carbonate in nickel-based plasmonic core@shell hybrid nanostructure for photocatalytic water splitting. Appl. Energy 2022, 322, 119461. [Google Scholar] [CrossRef]
- Ullah, I.; Ling, C.; Li, J.-H.; Lu, X.-J.; Li, C.; Yang, Z.; Qian, X.-J.; Wang, G.; Xu, A.-W. Metallic plasmons significantly boosted visible-light photocatalytic hydrogen evolution from water splitting. Sustain. Energy Fuels 2023, 7, 263–269. [Google Scholar] [CrossRef]
- Matsko, N.L. Formation of normal surface plasmon modes in small sodium nanoparticles. Phys. Chem. Chem. Phys. 2020, 22, 13285–13291. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Giangregorio, M.M.; Palumbo, F.; González, F.; Brown, A.S.; Moreno, F.; Losurdo, M. Sustainable and Tunable Mg/MgO Plasmon-Catalytic Platform for the Grand Challenge of SF6 Environmental Remediation. Nano Lett. 2020, 20, 3352–3360. [Google Scholar] [CrossRef]
- Hopper, E.R.; Boukouvala, C.; Asselin, J.; Biggins, J.S.; Ringe, E. Opportunities and Challenges for Alternative Nanoplasmonic Metals: Magnesium and Beyond. J. Phys. Chem. C 2022, 126, 10630–10643. [Google Scholar] [CrossRef] [PubMed]
- Hopper, E.R.; Wayman, T.M.R.; Asselin, J.; Pinho, B.; Boukouvala, C.; Torrente-Murciano, L.; Ringe, E. Size Control in the Colloidal Synthesis of Plasmonic Magnesium Nanoparticles. J. Phys. Chem. C 2022, 126, 563–577. [Google Scholar] [CrossRef]
- Gordillo, N.; Catalán-Gómez, S.; Pau, J.L.; Redondo-Cubero, A. Spectrally broad plasmonic absorption in Ga and In nanoparticle hybrids. Nanotechnology 2019, 30, 475705. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chien, C.-Y.; Wang, C.-M.; Lin, R.-S.; Chen, I.C. Plasmon Tuning of Liquid Gallium Nanoparticles through Surface Anodization. Materials 2022, 15, 2145. [Google Scholar] [CrossRef]
- Lian, Z.; Wu, F.; Zhong, Y.; Zi, J.; Li, Z.; Wang, X.; Nakagawa, T.; Li, H.; Sakamoto, M. Tuning plasmonic p–n junction for efficient infrared-light-responsive hydrogen evolution. Appl. Catal. B Environ. 2022, 318, 121860. [Google Scholar] [CrossRef]
- Li, N.; Fan, H.; Zhao, W.; Gao, Y.; Ge, L. 2D/0D plasmonic CuSe/CdS for efficient photocatalytic hydrogen activity via strong Vis-NIR light and interfacial effect. Appl. Surf. Sci. 2022, 590, 153028. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Huang, M.; Li, Q.; Zhao, L.; Sang, Y.; Zhang, X.; Zhao, Z.; Liu, H.; Zhou, W. Oxygen vacancy-regulated metallic semiconductor MoO2 nanobelt photoelectron and hot electron self-coupling for photocatalytic CO2 reduction in pure water. Appl. Catal. B Environ. 2022, 319, 121887. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, D.; Yan, Z.; Sun, Z.; Zhang, Z.; Xu, D.; Qiao, C.; Chen, Z.; Jia, Y.; Chan Jun, S.; et al. Interfacial coupled engineering of plasmonic amorphous MoO3−x nanodots/g-C3N4 nanosheets for photocatalytic water splitting and photothermal conversion. Chem. Eng. J. 2023, 453, 139875. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, K.; Zhang, J.; Dawson, G. Photocatalytic CO2 conversion of W18O49/CdSe-Diethylenetriamine with high charge transfer efficiency: Synergistic effect of LSPR effect and S-scheme heterojunction. Chin. J. Catal. 2022, 43, 2539–2547. [Google Scholar] [CrossRef]
- Ji, S.; Dong, J.; Ji, M.; Zou, W.; Yin, S.; Chen, Z.; Xia, J. Rapid dual-channel electrons transfer via synergistic effect of LSPR effect and build-in electric field in Z-scheme W18O49/BiOBr heterojunction for organic pollutants degradation. Inorg. Chem. Commun. 2022, 138, 109283. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, X.; Ma, Z.; Li, Y.; Yue, S.; Liu, X.; Zhang, J. W5+–W5+ Pair Induced LSPR of W18O49 to Sensitize ZnIn2S4 for Full-Spectrum Solar-Light-Driven Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2022, 32, 2203638. [Google Scholar] [CrossRef]
- He, X.; Liu, Q.; Xu, D.; Wang, L.; Tang, H. Plasmonic TiN nanobelts assisted broad spectrum photocatalytic H2 generation. J. Mater. Sci. Technol. 2022, 116, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, L.; Duan, A.; Zhang, Y.; Xiao, F.; Zhu, Y.; Wang, J.; Feng, C.; Yin, N. Adjusting charge kinetics of conjugated polymers via integration of LSPR effect with homojunction. Chem. Eng. J. 2023, 452, 139068. [Google Scholar] [CrossRef]
- Tan, X.; Wang, L.; Cheng, C.; Yan, X.; Shen, B.; Zhang, J. Plasmonic MoO3−x@MoO3 nanosheets for highly sensitive SERS detection through nanoshell-isolated electromagnetic enhancement. Chem. Commun. 2016, 52, 2893–2896. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Karabiyik, B.; Wang, K.; Wei, Z.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Vis-Responsive Copper-Modified Titania for Decomposition of Organic Compounds and Microorganisms. Catalysts 2020, 10, 1194. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Niu, C.-G.; Guo, H.; Huang, D.-W.; Liang, C.; Yang, Y.-Y.; Tang, N.; Zhang, X.-G. Integrating the Z-scheme heterojunction and hot electrons injection into a plasmonic-based Zn2In2S5/W18O49 composite induced improved molecular oxygen activation for photocatalytic degradation and antibacterial performance. J. Colloid Interface Sci. 2022, 610, 953–969. [Google Scholar] [CrossRef] [PubMed]



| Materials | Preparation Method | Origin of LSPR | Structure | Light Source | Objects | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Bi@SiO2 (Bi:SiO2 = 1:0.1) | Liquid phase chemical reduction method | Bi | Core–shell | Iodine lamp | RhB(10 ppm) | Degradation, 44.35% in 60 min | [29] |
| BPA(20 ppm) | Degradation, 49.10% in 60 min | ||||||
| Bi@SiO2 (Bi:SiO2 = 1:0.3) | RhB(10 ppm) | Degradation, 63.32% in 60 min | |||||
| BPA(20 ppm) | Degradation, 40.0% in 60 min | ||||||
| Bi-NPs@GO | A solution-based sonication method | Bi | Sphere | UV lamp | NO(100 ppm) | Removal, 42.3% in 30 min | [30] |
| C/Bi/Bi2O3 | One-pot method | Bi | — | Xe lamp (visible light + simulated sunlight) | 2,4-DCP(10 mg/L) | — | [31] |
| Bio/Bi2O3@C | A surfactant-assisted sol-gel method | Bi | — | LED daylight bulb | MB(10 mg/L) | Degradation, 75.0% in 60 min | [32] |
| Bi/Bi2WO6 | Hydrothermal reaction + solvothermal method | Bi | Hierarchical architecture | Visible light | RhB(10 mg/L) | Degradation, 93.0% in 25 min | [33] |
| 4-CP(20 mg/L) | Degradation, 54.4% in 120 min | ||||||
| g-C3N4@Bi@Bi2WO6 | Hydrothermal method | Bi | Flower-like hierarchical microspheres | Xe lamp | MO(10 mg/L) | Degradation, 70% in 120 min | [34] |
| RhB(20 mg/L) | Degradation, 100% in 30 min | ||||||
| 2,4-DCP(20 mg/L) | Degradation, 72% in 180 min | ||||||
| Bi@Bi4O7I/rGO | Surface-charge-mediated self-assembly strategy | Bi | 2D nanosheet | Visible light | LVFX(20 mg/L) | Degradation, 45.24% in 120 min | [35] |
| Bi NPs/BiOCl/ZnSn(OH)6 (ZSH-1-Bi) | One-pot precipitation, hydrolysis, and UV-photoreduction process | Bi | Cubes | Visible light | RhB (48 mg/L) | Degradation, 83.5% in 120 min | [36] |
| Bi NPs/BiOCl/ZnSn(OH)6 (ZSH-2-Bi) | 14-facets polyhedron | Degradation, 65.6% in 120 min | |||||
| Bi NPs/BiOCl/ZnSn(OH)6 (ZSH-3-Bi) | Octahedron | Degradation, 91.4% in 120 min | |||||
| Bi4MoO9/Bio | Hydrothermal method | Bi | Core–shell | Tungsten halogen lamp | NO(100 ppm) | Removal, 57.2% | [38] |
| Bi@BiOCl | One-step solvothermal method | Bi | Hierarchical microsphere | Tungsten halogen lamp | NO(100 ppm) | Removal, 67.5% | [39] |
| Bi-Bi2S3-g-C3N4 | Solvothermal method | Bi | PG structure | Visible light | Cr(VI) (20 mg/L) | Removal, 0.03921 min−1 | [40] |
| TC(10 mg/L) | Degradation, 92.5% in 150 min | ||||||
| Bi/(BiO)2CO3 | — | Bi | Staked irregular nanoplates | Tungsten halogen lamp | NO(100 ppm) | Removal, 63.6% in 30 min | [41] |
| BC/Bi/Fe3O4 | High-temperature calcination | Bi | The rod covered with nanoparticles | Xe lamp | Cr(VI) (20 mg/L) | Removal, 95% in 180 min | [43] |
| Cu2O/Bi/Bi2MoO6 | Two-step hydrothermal route + wet-impregnation | Bi | — | Visible light | Sulfadiazine (10 mg/L) | Degradation, 98.6% in 100 min | [44] |
| Ni(II)(10 mg/L) | Degradation, 93.2% in 60 min | ||||||
| g-C3N4/Bi/Bi3.64Mo0.36O6.55 | In situ reduction technique | Bi | — | Xe lamp | lomefloxacin (10 mg/L) | Degradation, 94.6% in 20 min | [48] |
| Materials | Preparation Method | Origin of LSPR | Structure | Light Source | Objects | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Cu/graphene (Cu/CG-1100) | In situ synthesis method | Cu | Staked layers of nanosheets | Xe lamp | H2 | Evolution, 3.94 mmol·g−1·h−1 | [50] |
| CuCo | Hydrothermal method | Cu | Dendrite-like | Xe lamp | H2 | Evolution, 77.1 umol·g−1·h−1 | [51] |
| Cu/TiO2 | Hydrothermal method | Cu | Nanorods | UVA lamps | CO2 | Reduction, 2.91 ppm/gcatal | [52] |
| Cu film/Cu2O/TiNT | Anodization combined with electrodeposition method | Cu | — | Xe lamp | MB(10 mg/L) | Degradation, 100% in 30 min | [53] |
| Cu/UiO-66 | Double-solvent approach + one-step reduction | Cu | Octahedrons | Xe lamp | Benzyl alcohol | Oxidation, 53.3% | [54] |
| Cu/rGO | In situ photoreduction process | Cu | Nanosheets | Xe lamp | H2, | Evolution, 59 mmol·g−1·h−1 | [55] |
| Cu@Cu2O/SiO2 | Precipitation and sol-gel methods | Cu | Cubic shapes | Sunlight | CIP(2 mg/L) | Degradation, 94.24% in 60 min | [57] |
| Materials | Preparation Method | Origin of LSPR | Structure | Light Source | Objects | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2/W18O49 | Two-step solvothermal procedure | W18O49 | Sheet-like | Full spectrum | RhB | Degradation, 95.2% in 50 min | [75] |
| Cu2−xSe-g-C3N4 | Solvent–thermal method | Cu2−xSe | — | Xe lamp | MB(2 × 10−5 M) | Degradation, 96.4% in 120 min | [88] |
| Cu1.8Se/Cu3Se2 | Precipitation method | Cu2−xSe | A phase junction structure | Xe lamp(Vis and NIR) | MO(50 mg/L) | Degradation, 82% in 120 min | [91] |
| WO3−x | One-step template-free hydrothermal route | WO3−x | Staked morphology of nanosheets | Hg lamp (UV) | RhB(2 × 10−5 M) | Degradation, 100% in 80 min | [92] |
| visible light | RhB(2 × 10−5 M) | Degradation, 77% in 320 min | |||||
| W18O49/g-C3N4 | Solvothermal method | W18O49 | Nanorod bundles | Simulated sunlight | H2 | Evolution, 15.2 umol·h−1 | [93] |
| CdS/WO3−x | Photoinduced electron injection | WO3−x | Bundles and nanowires | Visible-NIR light | H2 | Evolution, 1.6 mmol·g−1·h−1 | [94] |
| MoO3−x | Solvothermal method | MoO3−x | Sheet-like | Xe lamp | MO (20 mg/L) | Degradation, 95.4% in 120 min | [97] |
| CdS/MoO3−x | One-pot hydrothermal method | MoO3−x | — | Visible light | MB (10 mgL−1) | Degradation, 97.6% | [98] |
| TC (10 mg L−1) | Degradation, 85.5% | ||||||
| IO-TiO2-MoO3−x | Emulsion polymerization reaction + solvothermal method + calcination | MoO3−x | Honeycomb-like | Xe lamp | H2 | Evolution, 886 umol/(g·h) | [102] |
| RhB | Degradation, 90% in 60 min | ||||||
| MoO3−x/g-C3N4 | Hydrothermally in situ growing | MoO3−x | — | Xe lamp | H2 | Evolution 22.8 umol/h | [103] |
| ZnO | Low-temperature chemical etching method | ZnO | Nanoplates | UV | RhB | Degradation, 90% in 95 min | [106] |
| Nanobowls | Degradation, 90% in 83 min | ||||||
| Nanorings | Degradation, 90% in 81 min | ||||||
| ZnO/rGO | One-step low-temperature chemical etching route | ZnO | Nanoplates | Xe lamp | RhB (2 × 10−5 M) | Degradation, 90% in 140 min | [107] |
| Nanobowls | Degradation, 86% in 140 min | ||||||
| Nanorings | Degradation, 97% in 140 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, X.; Chen, M. Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications. Catalysts 2023, 13, 940. https://doi.org/10.3390/catal13060940
Li R, Wang X, Chen M. Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications. Catalysts. 2023; 13(6):940. https://doi.org/10.3390/catal13060940
Chicago/Turabian StyleLi, Rou, Xianfeng Wang, and Ming Chen. 2023. "Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications" Catalysts 13, no. 6: 940. https://doi.org/10.3390/catal13060940
APA StyleLi, R., Wang, X., & Chen, M. (2023). Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications. Catalysts, 13(6), 940. https://doi.org/10.3390/catal13060940