Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells
Abstract
:1. Introduction
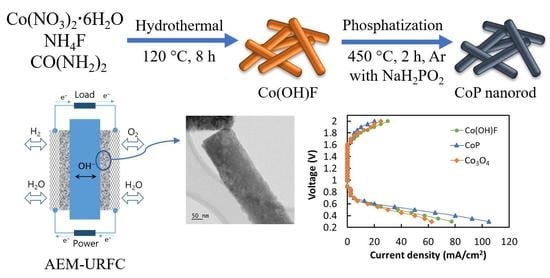
2. Results and Discussion
| Catalyst (mg cm−2) | Membrane | FC | WE | Ref. | |
|---|---|---|---|---|---|
| BHE | BOE | ||||
| Pt/C (2.0) | Co(OH)F (2.0) | FAA-3-50 | 77 (0.3 V) | 30 (2.0 V) | This work |
| Pt/C (2.0) | CoP (2.0) | FAA-3-50 | 105 (0.3 V) | 20 (2.0 V) | |
| Pt/C (2.0) | Co3O4 (2.0) | FAA-3-50 | 62 (0.3 V) | 25 (2.0 V) | |
| Pt/C (2.0) | Ir-black (2.0) | FAA-3-50 | 117 (0.3 V) | 50 (2.0 V) | |
| Pt/C (0.5) | MnOx-SS (0.3) | FAA-3-PK-130 | 65 (0.4 V) | 58 (1.7 V) | [45] |
| Pt/C (0.5) | MnOx/Ni-CP (0.3) | FAA-3-PK-130 | 65 (0.4 V) | 20 (1.8 V) | |
| Pt/C (0.5) | Fe-N-C + NiFe-LDH/C (0.5) | A201 | 95 (0.3 V) | 90 (1.8 V) | [46] |
| Ni/C (6.0) | Ni/C + MnOx/GC (4.0) | FAA3 | 24 (0.4 V) | 17 (1.8 V) | [47] |
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Yan, L.; Luo, H.; Mustain, W.; Xu, H. Recent progress and perspectives of bifunctional oxygen reduction/evolution catalyst development for regenerative anion exchange membrane fuel cells. Nano Energy 2018, 47, 172–198. [Google Scholar] [CrossRef]
- Chen, G.; Bare, S.R.; Mallouk, T.E. Development of supported bifunctional electrocatalysts for unitized regenerative fuel cells. J. Electrochem. Soc. 2002, 149, A1092. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.H.; Kim, T.H.; Park, K.W.; Jung, S.; Kurkuri, M.D.; Jung, H.Y. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrogen Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Ul Hassan, N.; Ganesan, P.; Lando, A.A.; Mustain, W.E.; Colón-Mercado, H.R. Stable, high-performing bifunctional electrodes for anion exchange membrane-based unitized regenerative fuel cells. J. Power Sources 2022, 541, 231599. [Google Scholar] [CrossRef]
- Shrestha, S.; Liu, Y.; Mustain, W.E. Electrocatalytic activity and stability of Pt clusters on state-of-the-art supports: A review. Catal. Rev. Sci. Eng. 2011, 53, 256–336. [Google Scholar] [CrossRef]
- Setzler, B.P.; Zhuang, Z.; Wittkopf, J.A.; Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 2016, 11, 1020–1025. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Trocino, S.; Aricò, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Du, L.; Xing, L.; Zhang, G.; Dubois, M.; Sun, S. Strategies for engineering high-performance PGM-free catalysts toward oxygen reduction and evolution reactions. Small Methods 2020, 4, 2000016. [Google Scholar] [CrossRef]
- Yang, M.; Xie, J.Y.; Lin, Z.Y.; Dong, B.; Chen, Y.; Ma, X.; Wen, M.L.; Zhou, Y.N.; Wang, L.; Chai, Y.M. N-doped FeP nanorods derived from Fe-MOFs as bifunctional electrocatalysts for overall water splitting. Appl. Surf. Sci. 2020, 507, 145096. [Google Scholar] [CrossRef]
- Xie, X.Q.; Liu, J.; Gu, C.; Li, J.; Zhao, Y.; Liu, C.S. Hierarchical structured CoP nanosheets/carbon nanofibers bifunctional eletrocatalyst for high-efficient overall water splitting. J. Energy Chem. 2022, 64, 503–510. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zou, R.; Shi, G.; Huang, Y.; Yang, W.; Yang, W.; Liu, C.; Peng, X. Coupling overall water splitting and biomass oxidation via Fe-doped Ni2P@ C nanosheets at large current density. Appl. Catal. B Environ. 2022, 307, 121170. [Google Scholar] [CrossRef]
- Sun, D.; Lin, S.; Yu, Y.; Liu, S.; Meng, F.; Du, G.; Xu, B. One-pot synthesis of N and P Co-doped carbon layer stabilized cobalt-doped MoP 3D porous structure for enhanced overall water splitting. J. Alloys Compd. 2022, 895, 162595. [Google Scholar] [CrossRef]
- Abdullah, U.; Ali, M.; Pervaiz, E.; Khosa, R. An inclusive perspective on the recent development of tungsten-based catalysts for overall water-splitting: A review. Int. J. Energy Res. 2022, 46, 10228–10258. [Google Scholar] [CrossRef]
- Chang, J.; Xiao, Y.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015, 5, 6874–6878. [Google Scholar] [CrossRef]
- Shang, F.; Yu, W.; Shi, R.; Wan, S.; Zhang, H.; Wang, B.; Cao, R. Enhanced lithium storage performance guided by intricate-cavity hollow cobalt phosphide. Appl. Surf. Sci. 2021, 563, 150395. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Ran, S.; Huang, H.; Wang, X.; Liang, B.; Chen, D.; Shen, G. Nanorod-assembled Co3O4 hexapods with enhanced electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 23541–23546. [Google Scholar] [CrossRef]
- He, K.; Tadesse Tsega, T.; Liu, X.; Zai, J.; Li, X.H.; Liu, X.; Li, W.; Ali, N.; Qian, X. Utilizing the space-charge region of the FeNi-LDH/CoP p-n junction to promote performance in oxygen evolution electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 11903–11909. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Z.; Meng, S.; Yu, R.; Jiang, D.; Chen, M. Iron and chromium co-doped cobalt phosphide porous nanosheets as robust bifunctional electrocatalyst for efficient water splitting. Nanotechnology 2021, 33, 075204. [Google Scholar] [CrossRef]
- Yang, H.; Driess, M.; Menezes, P.W. Self-supported electrocatalysts for practical water electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Xu, T.; Yang, L.; Li, J.; Usoltseva, N.; An, V.; Jin, X.; Zhang, C.; Zhang, X.; Liu, B. NH4F-induced morphology control of CoP nanostructures to enhance the hydrogen evolution reaction. Inorg. Chem. 2021, 60, 10781–10790. [Google Scholar] [CrossRef]
- Abbas, S.K.; Atiq, S.; Saleem, M.; Riaz, S.; Naseem, S.; Anwar, M.S. Fluoride ion assisted growth of hierarchical flowerlike nanostructures of Co/Ni ferrites and their magnetoresistive response. RSC Adv. 2019, 9, 17581–17590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Fang, J.; Wang, X.Y.; Yin, L.; Zhu, W.; Zhuang, Z. Cr-Doped CoP Nanorod Arrays as High-Performance Hydrogen Evolution Reaction Catalysts at High Current Density. Small 2021, 17, 2100832. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Liang, L.; Li, C.; Wang, M.; Ge, J.; Liu, C.; Xing, W. Ultrathin cobalt phosphide nanosheets as efficient bifunctional catalysts for a water electrolysis cell and the origin for cell performance degradation. Green Chem. 2016, 18, 2287–2295. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Wang, X.; Zhang, D.; Chen, S. Three-dimensional metal–organic framework derived porous CoP 3 concave polyhedrons as superior bifunctional electrocatalysts for the evolution of hydrogen and oxygen. Phys. Chem. Chem. Phys. 2017, 19, 2104–2110. [Google Scholar] [CrossRef]
- Li, R.; Hu, B.; Yu, T.; Chen, H.; Wang, Y.; Song, S. Insights into correlation among surface-structure-Activity of cobalt-derived pre-catalyst for oxygen evolution reaction. Adv. Sci. 2020, 7, 1902830. [Google Scholar] [CrossRef]
- Li, Z.; Zou, Y.; Duan, J.; Long, B. Coral-like CoP hollow composites as effective host cathodes for lithium-sulfur batteries. Ionics 2019, 25, 4625–4635. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.C.; Chang, Y.C.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Z.; Li, Z.; Yang, Y.; Zhao, J.; Zhu, Y.; Miao, W. Cobalt phosphide embedded N-doped carbon nanopolyhedral as an efficient cathode electrocatalyst in microbial fuel cells. J. Environ. Chem. Eng. 2021, 9, 104582. [Google Scholar] [CrossRef]
- Niu, H.J.; Wang, A.J.; Zhang, L.; Feng, J.J. Bioinspired One-Step Pyrolysis Fabrication of 3D Porous Co, N, P-doped Carbon Nanosheets with Enriched CoNx Active Sites as High-Performance Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. ACS Appl. Energy Mater. 2020, 3, 2781–2790. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Feng, Y.; Yuan, Q.; Gao, J.; Chen, Y.; Huang, Y.; He, Y.-B.; Gan, W. Microwave-assisted synthesis of carbon nanotubes threaded core-shell CoPx/Co-Nx-C@CNT and its performance as an efficient bifunctional oxygen catalyst for the rechargeable zinc-air battery. Mater. Today Phys. 2019, 9, 100132. [Google Scholar] [CrossRef]
- Pei, Y.; Cheng, Y.; Chen, J.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Recent developments of transition metal phosphides as catalysts in the energy conversion field. J. Mater. Chem. A 2018, 6, 23220–23243. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zheng, L.; Chen, Z.; Wu, Z.; Sun, L.; Hu, Z.; Liu, X. CoO/CoP heterostructured nanosheets with an O–P interpenetrated interface as a bifunctional electrocatalyst for Na–O2 battery. ACS Catal. 2018, 8, 8953–8960. [Google Scholar] [CrossRef]
- Lyu, C.; Cheng, J.; Wu, K.; Wu, J.; Hao, J.; Chen, Y.; Wang, H.; Yang, Y.; Wang, N.; Lau, W.-M.; et al. Interface and cation dual-engineering promoting Ce-Co(OH)2/CoP/NF as bifunctional electrocatalyst toward overall water splitting coupling with oxidation of organic compounds. J. Alloys Compd. 2023, 934, 167942. [Google Scholar] [CrossRef]
- Liu, X.; Wei, B.; Su, R.; Zhao, C.; Dai, D.; Ma, X.; Xu, L. Mo-Doped Cobalt Phosphide Nanosheets for Efficient Hydrogen Generation in an Alkaline Media. Energy Technol. 2019, 7, 1900021. [Google Scholar] [CrossRef]
- Chen, T.; Qin, S.; Qian, M.; Dai, H.; Fu, Y.; Zhang, Y.; Ye, B.; Lin, Q.; Yang, Q. Defect-Rich Fe-Doped CoP Nanosheets as Efficient Oxygen Evolution Electrocatalysts. Energy Fuels 2021, 35, 10890–10897. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Sun, Q.; Huang, W.-H.; Wang, Z.; Chueh, C.-C.; Chen, C.-L.; Zhu, Z. Surface engineered CoP/Co3O4 heterojunction for high-performance bi-functional water splitting electro-catalysis. Nanoscale 2021, 13, 20281–20288. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Daiyan, R.; Leverett, J.; Fusco, Z.; Tadich, A.; Di Bernardo, I.; Kiy, A.; Truong, T.N.; Zhang, Q.; Chen, H.; et al. Understanding the activity and stability of flame-made Co3O4 spinels: A route towards the scalable production of highly performing OER electrocatalysts. Chem. Eng. J. 2022, 429, 132180. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Perumal, S.; Seo, J. Enhanced alkaline water splitting on cobalt phosphide sites by 4d metal (Rh)-doping method. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Alsabban, M.M.; Eswaran, M.K.; Peramaiah, K.; Wahyudi, W.; Yang, X.; Ramalingam, V.; Hedhili, M.N.; Miao, X.; Schwingenschlögl, U.; Li, L.J.; et al. Unusual activity of rationally designed cobalt phosphide/oxide heterostructure composite for hydrogen production in alkaline medium. ACS Nano 2022, 16, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhou, H.; Zhu, Z.; Sun, J.; He, R.; Bao, J.; Chen, S.; Ren, Z. Three-dimensional nanoporous iron nitride film as an efficient electrocatalyst for water oxidation. ACS Catal. 2017, 7, 2052–2057. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.; Li, S.; Gu, Y.; Lan, J.; Zhou, Q.; Xu, W. Oxygen vacancy-rich ultrafine CoP/Co3O4 nanoparticles as high-efficiency trifunctional electrocatalyst. Electrochim. Acta 2022, 412, 140134. [Google Scholar] [CrossRef]
- Ng, J.W.D.; Tang, M.; Jaramillo, T.F. A carbon-free, precious-metal-free, high-performance O2 electrode for regenerative fuel cells and metal–air batteries. Energy Environ. Sci. 2014, 7, 2017–2024. [Google Scholar] [CrossRef]
- Dresp, S.; Luo, F.; Schmack, R.; Kühl, S.; Gliech, M.; Strasser, P. An efficient bifunctional two-component catalyst for oxygen reduction and oxygen evolution in reversible fuel cells, electrolyzers and rechargeable air electrodes. Energy Environ. Sci. 2016, 9, 2020–2024. [Google Scholar] [CrossRef]
- Desmond Ng, J.W.; Gorlin, Y.; Hatsukade, T.; Jaramillo, T.F. A precious-metal-free regenerative fuel cell for storing renewable electricity. Adv. Energy Mater. 2013, 3, 1545–1550. [Google Scholar] [CrossRef]
- Rana, M.M.; Park, G.; Sun, H.J.; Rim, H.R.; Lee, H.K.; Shim, J. Cell performance and polarization analysis on different operating conditions in anion exchange membrane-unitized regenerative fuel cells (AEM-URFCs). Korean J. Chem. Eng. 2022, 39, 3295–3304. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, P.; Rana, M.M.; Kang, B.-S.; Sun, H.-J.; Park, G.; Kim, S.-Y.; Lee, H.-K.; Shim, J. Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells. Catalysts 2023, 13, 941. https://doi.org/10.3390/catal13060941
Rajkumar P, Rana MM, Kang B-S, Sun H-J, Park G, Kim S-Y, Lee H-K, Shim J. Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells. Catalysts. 2023; 13(6):941. https://doi.org/10.3390/catal13060941
Chicago/Turabian StyleRajkumar, Palanisamy, Md. Masud Rana, Beom-Soo Kang, Ho-Jung Sun, Gyungse Park, So-Yeon Kim, Hong-Ki Lee, and Joongpyo Shim. 2023. "Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells" Catalysts 13, no. 6: 941. https://doi.org/10.3390/catal13060941
APA StyleRajkumar, P., Rana, M. M., Kang, B. -S., Sun, H. -J., Park, G., Kim, S. -Y., Lee, H. -K., & Shim, J. (2023). Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells. Catalysts, 13(6), 941. https://doi.org/10.3390/catal13060941