Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
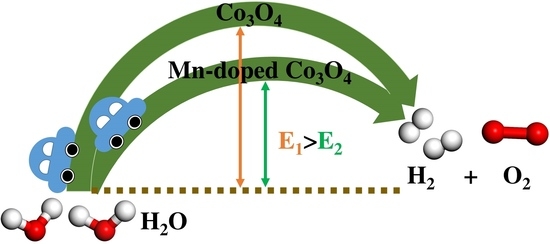
2.1. DFT Calculation Results
2.2. Electrode Preparation and Characterization
3. Materials and Methods
3.1. Theoretical Calculations
3.2. Preparation and Testing of Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Yun, S.; Wu, L.; Zhang, J.; Shi, X.; Wei, W.; Liu, Y.; Zheng, R.; Han, N.; Ni, B.-J. Waste-Derived Catalysts for Water Electrolysis: Circular Economy-Driven Sustainable Green Hydrogen Energy. Nano-Micro Lett. 2023, 15, 4. [Google Scholar]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [PubMed]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of noble metals—A review. Chemosphere 2022, 286, 131805. [Google Scholar] [PubMed]
- Wang, J.; Liao, T.; Wei, Z.; Sun, J.; Guo, J.; Sun, Z. Heteroatom-Doping of Non-Noble Metal-Based Catalysts for Electrocatalytic Hydrogen Evolution: An Electronic Structure Tuning Strategy. Small Methods 2021, 5, 2000988. [Google Scholar]
- Wang, C.; Shang, H.; Xu, H.; Du, Y. Nanoboxes endow non-noble-metal-based electrocatalysts with high efficiency for overall water splitting. J. Mater. Chem. A 2021, 9, 857–874. [Google Scholar]
- Zhou, Q.; Liao, L.; Zhou, H.; Li, D.; Tang, D.; Yu, F. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Mater. Today Phys. 2022, 26, 100727. [Google Scholar]
- Xu, X.M.; Shao, Z.P.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Poudel, M.B.; Logeshwaran, N.; Kim, A.R.; Karthikeyan, S.C.; Vijayapradeep, S.; Yoo, D.J. Integrated core-shell assembly of Ni3S2 nanowires and CoMoP nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Alloys Compd. 2023, 960, 170678. [Google Scholar] [CrossRef]
- Dong, Y.; Deng, Z.; Xu, Z.; Liu, G.; Wang, X. Synergistic Tuning of CoO/CoP Heterojunction Nanowire Arrays as Efficient Bifunctional Catalysts for Alkaline Overall Water Splitting. Small Methods 2023, 2300071. [Google Scholar] [CrossRef]
- Shah, A.A.; Kumar, M.; Aftab, U.; Abro, M.I. Seawater-Extracted MgO-Doped Co3O4 Composite for Electrochemical Water Splitting. Chem. Eng. Technol. 2023, 46, 459–465. [Google Scholar] [CrossRef]
- Sai, K.N.S.; Tang, Y.; Dong, L.; Yu, X.-Y.; Hong, Z. N2 plasma-activated NiO nanosheet arrays with enhanced water splitting performance. Nanotechnology 2020, 31, 455709. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, X.; Zhu, B.; Li, G.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C. Redox route to ultrathin metal sulfides nanosheet arrays-anchored MnO2 nanoparticles as self-supported electrocatalysts for efficient water splitting. J. Power Sources 2018, 398, 159–166. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.F.; Zhang, L.; Zu, M.Y.; Yang, Y.X.; Yang, H.G. Enhancing alkaline hydrogen evolution reaction activity through Ni-Mn3O4 nanocomposites. Chem. Commun. 2016, 52, 10566. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Ji, J.-Y.; Niu, Z.; Gu, H.; Abrahams, B.F.; Lang, J.-P. Tailorable carbon cloth electrodes covered with heterostructured Co/CoO/CoN interfaces for scalable electrocatalytic overall water splitting. Chem. Eng. J. 2023, 461, 141937. [Google Scholar]
- Yang, W.; Wei, A.; Liu, J.; Xiao, Z.; Zhao, Y.; Zhang, Y. Rational construction of vertical few layer graphene/NiO core-shell nanoflake arrays for efficient oxygen evolution reaction. Mater. Res. Bull. 2021, 139, 111260. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ma, N.; Yin, Z.; Yang, X.; Yin, Z.; Gao, J.; Xu, Y.; Gao, Z.; Wang, H.; Kang, J.; et al. Rich Surface Oxygen Vacancies of MnO2 for Enhancing Electrocatalytic Oxygen Reduction and Oxygen Evolution Reactions. Adv. Energy Sustain. Res. 2021, 2, 2100030. [Google Scholar] [CrossRef]
- Wang, Q.; Dastafkan, K.; Zhao, C. Design strategies for non-precious metal oxide electrocatalysts for oxygen evolution reactions. Curr. Opin. Electrochem. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Boppella, R.; Tan, J.; Yun, J.; Manorama, S.V.; Moon, J. Anion-mediated transition metal electrocatalysts for efficient water electrolysis: Recent advances and future perspectives. Coord. Chem. Rev. 2021, 427, 213552. [Google Scholar]
- Poudel, M.B.; Ojha, G.P.; Kim, A.A.; Kim, H.J. Manganese-doped tungsten disulfide microcones as binder-free electrode for high performance asymmetric supercapacitor. J. Energy Storage 2022, 47, 103674. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Synthesis of high-performance nickel hydroxide nanosheets/gadolinium doped-α-MnO2 composite nanorods as cathode and Fe3O4/GO nanospheres as anode for an all-solid-state asymmetric supercapacitor. J. Energy Chem. 2022, 64, 475–484. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, W.-J.; Niu, S.; Liu, N.; Luo, H.; Chen, Y.-Y.; Jin, S.-F.; Gao, F.; Wan, L.-J.; Hu, J.-S. Electronic and Morphological Dual Modulation of Cobalt Carbonate Hydroxides by Mn Doping toward Highly Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. J. Am. Chem. Soc. 2017, 139, 8320–8328. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Yin, H.; You, T.; Zheng, L.; Zhang, J.; Wang, P.; Jin, Z.; Tian, Y.; Liu, J.; Tang, Z.; et al. Efficient Electrocatalytic Water Oxidation by Using Amorphous Ni-Co Double Hydroxides Nanocages. Adv. Energy Mater. 2015, 5, 1401880. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Tian, P.; Zhou, S.; Gao, H.; Su, J.; Tian, X.; Zang, J. Amorphous NiFe-OH/Ni-Cu-P supported on selfsupporting expanded graphite sheet as efficient bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energ. 2020, 45, 30387–30395. [Google Scholar] [CrossRef]
- Gugtapeh, H.S.; Rezaei, M. Facile electrochemical synthesis of Ni-Sb nanostructure supported on graphite as an affordable bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Electroanal. Chem. 2022, 922, 116726. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Li, Y.; Wu, J.; Wang, Q.; Yan, Z.; Li, C.-P.; Cheng, F.; Du, M. Coupling NiCo Alloy and CeO2 to Enhance Electrocatalytic Hydrogen Evolution in Alkaline Solution. Adv. Sustain. Syst. 2020, 4, 2000122. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Fan, G.; Qi, J.; Liu, Z.; Yan, Z.; Cheng, F.; Chen, J.; Li, C.-P.; Du, M. Boosting Activity on Co4N Porous Nanosheet by Coupling CeO2 for Efficient Electrochemical Overall Water Splitting at High Current Densities. Adv. Funct. Mater. 2020, 30, 1910596. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, X.; Zhao, F.; Xu, Q.; Xu, Y.; Xu, Y.; Chen, S.; Fan, X.; Qiao, X. Mono-crystalline Ge1−xSnxSe micro-sheets with hexagonal morphologies for Visible-NIR photodetectors: Increased carrier concentration, narrowed band gap and improved performances. J. Solid State Chem. 2022, 310, 123068. [Google Scholar] [CrossRef]
- Saad, A.A.; Lattief, F.A. Test of conductor and semiconductor electrocatalysts in high voltage alkaline electrolyzer as production media for green hydrogen. Int. J. Hydrogen Energ. 2023, 48, 1717–1732. [Google Scholar]
- Xuan, C.; Xu, Q.; Han, L.; Hou, B. Electronic structure exquisite modulation of NiSe2 interface via rationally controlling Fe doping for boosting electrochemical oxygen evolution activity. Chem. Eng. J. 2023, 464, 142620. [Google Scholar]
- Jiao, S.; Fu, X.; Huang, H. Descriptors for the Evaluation of Electrocatalytic Reactions: D-Band Theory and Beyond. Adv. Funct. Mater. 2022, 32, 2107651. [Google Scholar]
- Wang, J.; Xin, S.; Xiao, Y.; Zhang, Z.; Li, Z.; Zhang, W.; Li, C.; Bao, R.; Peng, J.; Yi, J.; et al. Manipulating the Water Dissociation Electrocatalytic Sites of Bimetallic Nickel-Based Alloys for Highly Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202202518. [Google Scholar]
- Hsiao, M.-C.; Liao, S.-H.; Yen, M.-Y.; Liu, P.-I.; Pu, N.-W.; Wang, C.-A.; Ma, C.-C.M. Preparation of Covalently Functionalized Graphene Using Residual Oxygen-Containing Functional Groups. ACS Appl. Mater. Interfaces 2010, 2, 3092–3099. [Google Scholar] [CrossRef]
- Aswathy, N.R.; Varghese, J.; Vinodkumar, R. Effect of annealing temperature on the structural, optical, magnetic and electrochemical properties of NiO thin films prepared by sol-gel spin coating. J. Mater. Sci.-Mater. Electron. 2020, 31, 16634–16648. [Google Scholar] [CrossRef]
- Bigiani, L.; Maccato, C.; Barreca, D.; Gasparotto, A. MnO2 nanomaterials functionalized with Ag and SnO2: An XPS study. Surf. Sci. Spectra 2020, 27, 024005. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A-Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Chen, X.; Liu, P.; Huang, X.; Wei, J.; Ren, Z.; Yao, S.; Fang, Z.; Wang, T.; Masa, J. Co-Mn Hybrid Oxides Supported on N-Doped Graphene as Efficient Electrocatalysts for Reversible Oxygen Electrodes. J. Electrochem. Soc. 2018, 165, H580–H589. [Google Scholar] [CrossRef]
- Nassar, M.A.; El-dek, S.I.; Rouby, W.M.A.E.; El-Deen, A.G. Highly efficient asymmetric supercapacitor-based on Ni-Co oxides intercalated graphene as positive and Fe2O3 doped graphene as negative electrodes. J. Energy Storage 2021, 44, 103305. [Google Scholar]
- Shan, X.; Guo, F.; Page, K.; Neuefeind, J.C.; Ravel, B.; Abeykoon, A.M.M.; Kwon, G.; Olds, D.; Su, D.; Teng, X. Framework Doping of Ni Enhances Pseudocapacitive Na-Ion Storage of (Ni)MnO2 Layered Birnessite. Chem. Mater. 2019, 31, 8774–8786. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhang, J.-Y.; Chen, Y.; Yang, X.; Song, W.; Wei, L.; Li, W. Synergy of Mn and Ni enhanced catalytic performance for toluene combustion over Ni-doped α-MnO2 catalysts. Chem. Eng. J. 2020, 388, 124244. [Google Scholar] [CrossRef]
- Huang, X.F.; Chang, S.; Wee, S.V.; Ding, J.; Xue, J.M. Three-dimensional printed cellular stainless steel as a high-activity catalytic electrode for oxygen evolution. J. Mater. Chem. A 2017, 5, 18176–18182. [Google Scholar] [CrossRef]
- Nørskov, J.K. Chemisorption on metal surfaces. Rep. Prog. Phys. 1990, 53, 1253–1295. [Google Scholar] [CrossRef]
- Nørskov, J.K. Electronic factors in catalysis. Prog. Surf. Sci. 1991, 38, 103–144. [Google Scholar] [CrossRef]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.J.; Yang, C. Exceptional performance of hierarchical Ni-Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Ding, Y.; Jin, H.; Zeng, T. Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting. Catalysts 2023, 13, 1031. https://doi.org/10.3390/catal13071031
Lin J, Ding Y, Jin H, Zeng T. Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting. Catalysts. 2023; 13(7):1031. https://doi.org/10.3390/catal13071031
Chicago/Turabian StyleLin, Jie, Yihong Ding, Huile Jin, and Tianbiao Zeng. 2023. "Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting" Catalysts 13, no. 7: 1031. https://doi.org/10.3390/catal13071031
APA StyleLin, J., Ding, Y., Jin, H., & Zeng, T. (2023). Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting. Catalysts, 13(7), 1031. https://doi.org/10.3390/catal13071031