Using a Surface-Response Approach to Optimize the Photocatalytic Activity of rGO/g-C3N4 for Bisphenol A Degradation
Abstract
:1. Introduction
2. Results
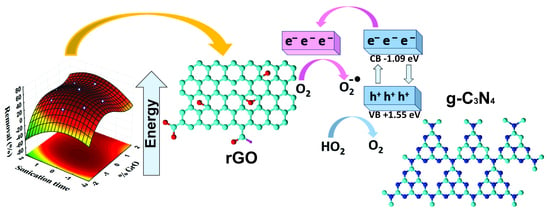
2.1. The rGO/g-C3N4 Preparation and Characterization
2.2. BPA Degradation Kinetics and Scavenging Tests
2.3. Degradation Product Identification and Ecotoxicity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of g-C3N4, rGO, and rGO/g-C3N4
3.3. Photodegradation Experiments
3.4. Identification of the DPs and Ecotoxicity Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padovan, R.N.; de Carvalho, L.S.; de Souza Bergo, P.L.; Xavier, C.; Leitão, A.; dos Santos Neto, A.J.; Lanças, F.M.; Azevedo, E.B. Degradation of hormones in tap water by heterogeneous solar TiO2-photocatalysis: Optimization, degradation products identification, and estrogenic activity removal. J. Environ. Chem. Eng. 2021, 9, 106442. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziv-Gal, A.; Flaws, J.A. Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertil. Steril. 2016, 106, 827–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassenaar, P.N.H.; Trasande, L.; Legler, J. Systematic review and meta-analysis of early-life exposure to bisphenol A and obesity-related outcomes in rodents. Environ. Health Perspect. 2017, 125, 106001-1–106001-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochester, J.R.; Bolden, A.L.; Kwiatkowski, C.F. Prenatal exposure to bisphenol A and hyperactivity in children: A systematic review and meta-analysis. Environ. Int. 2018, 114, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.L.; Kim, K.-H.; Kavitha, B.; Kumar, V.; Raza, V.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified titanium dioxide for photocatalytic applications. In Photocatalysts: Applications and Attributes, 1st ed.; Khan, S.B., Akhtar, K., Eds.; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Liu, C.; Zhang, Y.; Wu, J.; Dai, H.; Ma, C.; Zhang, Q.; Zou, Z. Ag-Pd alloy decorated ZnIn2S4 microspheres with optimal Schottky barrier height for boosting visible-light-driven hydrogen evolution. J. Mater. Sci. 2022, 114, 81–89. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Xie, K.; Li, X.; Liao, G. Photocatalytic degradation of organic pollutants over MoS2/Ag-ZnFe2O4 Z-scheme heterojunction: Revealing the synergistic effects of exposed crystal facets, defect engineering, and Z-scheme mechanism. Chem. Eng. J. 2023, 453, 139775. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yuan, Y.-P.; Fang, J.; Shahjamali, M.M.; Boey, F.Y.; Barber, J.; Loo, S.C.; Xue, C. In-situ growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation. Int. J. Hydrogen Energ 2013, 38, 1258–1266. [Google Scholar] [CrossRef]
- Ai, B.; Duan, X.; Sun, H.; Qiu, X.; Wang, S. Metal-free graphene-carbon nitride hybrids for photodegradation of organic pollutants in water. Catal. Today 2015, 258, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Low, J.; Long, R.; Xiong, Y. Metal-free electrocatalysts for nitrogen reduction reaction. EnergyChem 2020, 2, 100040. [Google Scholar] [CrossRef]
- He, F.; Xing, C.; Xue, Y. Metal-free amino-graphdiyne for applications in electrocatalytic hydrogen evolution. J. Catal. 2021, 395, 129–135. [Google Scholar] [CrossRef]
- Chen, K.; Chai, Z.; Li, C.; Shi, L.; Liu, M.; Xie, Q.; Zhang, Y.; Xu, D.; Manivannan, A.; Liu, Z. Catalyst-free growth of three-dimensional graphene flakes and graphene/g-C3N4 composite for hydrocarbon oxidation. ACS Nano 2016, 10, 3665–3673. [Google Scholar] [CrossRef]
- Cadan, F.M.; Ribeiro, C.; Azevedo, E.B. Improving g-C3N4:WO3 Z-scheme photocatalytic performance under visible light by multivariate optimization of g-C3N4 synthesis. Appl. Surf. Sci 2021, 537, 147904. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Sharma, M.; Madras, G.; Bose, S. A critical review on in situ reduction of graphene oxide during preparation of conducting polymeric nanocomposites. RSC Advances 2015, 5, 32078–32087. [Google Scholar] [CrossRef] [Green Version]
- Nikokavoura, A.; Trapalis, C. Alternative photocatalysts to TiO2 for the photocatalytic reduction of CO2. Appl. Surf. Sci. 2017, 391, 149–174. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Two dimensional graphitic materials for photoelectrocatalysis: A short review. Cat. Today 2018, 315, 2–8. [Google Scholar] [CrossRef]
- Hao, Q.; Hao, S.; Niu, X.; Li, X.; Chen, D.; Ding, H. Enhanced photochemical oxidation ability of carbon nitride by π–π stacking interactions with graphene. Chin. J Catal. 2017, 38, 278–286. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, Y.; Zou, J.; Shen, T.; Xu, Q.; Yue, X.; Meng, J.; Wang, J. The ultra-rapid synthesis of rGO/g-C3N4 composite via microwave heating with enhanced photo-catalytic performance. Mater. Lett. 2018, 232, 107–109. [Google Scholar] [CrossRef]
- Qian, J.; Yan, J.; Shen, C.; Xi, F.; Dong, X.; Liu, J. Graphene quantum dots-assisted exfoliation of graphitic carbon nitride to prepare metal-free zero-dimensional/two-dimensional composite photocatalysts. J. Mater. Sci. 2018, 53, 12103–12114. [Google Scholar] [CrossRef]
- Wan, J.; Pu, C.; Wang, R.; Liu, E.; Du, X.; Bai, X.; Fan, J.; Hu, X. A facile dissolution strategy facilitated by H2SO4 to fabricate a 2D metal-free g-C3N4/rGO heterojunction for efficient photocatalytic H2 production. Int. J. Hydrogen Energ. 2018, 43, 7007–7019. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, B.-K.; Hou, W.-C. Graphitic carbon nitride embedded with graphene materials towards photocatalysis of bisphenol A: The role of graphene and mediation of superoxide and singlet oxygen. Chemosphere 2021, 278, 130334. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrzak, M.; Kukulka, W.; Mijowska, E. Graphitic carbon nitride/graphene oxide/reduced graphene oxide nanocomposites for photoluminescence and photocatalysis. Appl. Surf. Sci. 2017, 398, 56–62. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B-Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. Fabrication of metal-free 2D/2D g-C3N4/rGO composite towards the degradation of harmful organics. Optik 2020, 219, 165023. [Google Scholar] [CrossRef]
- Bruns, R.E.; Scaramino, I.S.; Barros Neto, B. Statistical Design—Chemometrics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; p. 422. [Google Scholar]
- He, H.; Huang, L.; Zhong, Z.; Tan, S. Constructing three-dimensional porous graphene-carbon quantum dots/g-C3N4 nanosheet aerogel metal-free photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 441, 285–294. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, D.; Zhan, H. Construction of reduced graphene oxide nanofibers and cobalt sulfide nanocomposite for pseudocapacitors with enhanced performance. J. Alloy Compd. 2017, 706, 126–132. [Google Scholar] [CrossRef]
- Blanton, T.N.; Majumdar, D. Characterization of X-ray irradiated graphene oxide coatings using X-ray diffraction, X-ray photoelectron spectroscopy, and atomic force microscopy. Powder Diffr. 2013, 28, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Y.; Tang, Y.; Dai, S.; Qiao, S.Z. Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: An excellent metal-/label-free biosensing platform. Small 2014, 10, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-W.; Yu, H.-Y.; Yang, Y.-J.; Li, H.-J.; Zhai, C.-Y.; Qian, D.-J.; Chen, M. New complete assignment of X-ray powder diffraction patterns in graphitic carbon nitride using discrete Fourier transform and direct experimental evidence. Phys. Chem. Chem. Phys. 2017, 19, 26072–26084. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.; Carvalho, K.T.; Lopes, O.F.; Ribeiro, C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis: Enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation. Appl. Catal. B-Environ. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Malagutti, A.R.; Mourão, H.A.J.L.; Garbin, J.R.; Ribeiro, C. Deposition of TiO2 and Ag:TiO2 thin films by the polymeric precursor method and their application in the photodegradation of textile dyes. Appl. Catal. B Environ. 2009, 90, 205–212. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interf. Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef]
- Ji, H.; Chang, F.; Hu, X.; Qin, W.; Shen, J. Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chem. Eng. J. 2013, 218, 183–190. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Liu, M.; Zhao, X. Insights into heterogeneous catalytic activation of peroxymonosulfate by Pd/g-C3N4: The role of superoxide radical and singlet oxygen. Catal. Commun. 2017, 102, 85–88. [Google Scholar] [CrossRef]
- Bauer, R.; Fallmann, H. The photo-Fenton oxidation—A cheap and efficient wastewater treatment method. Res. Chem. Intermediat. 1997, 23, 341–354. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Xu, Z.; Loo, J.S.C. Hybrid catalysts for photoelectrochemical reduction of carbon dioxide: A prospective review on semiconductor/metal complex co-catalyst systems. J. Mater. Chem. 2014, 2, 15228–15233. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Alamgholiloo, H.; Noroozi Pesyan, N.; Zolfigol, M.A. A MOF-on-MOF strategy to construct double Z-scheme heterojunction for high-performance photocatalytic degradation. Appl. Catal. B 2023, 321, 122082. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Ignatev, A.N.; Frimmel, F.H.; Bräse, S.; Horn, H.; Revelsky, A.I. Formation of genotoxic quinones during bisphenol A degradation by TiO2 photocatalysis and UV photolysis: A comparative study. Appl. Catal. B Environ. 2014, 160–161, 106–114. [Google Scholar] [CrossRef]
- Li, K.Y.; Kuo, C.H.; Weeks Junior, J.L. A kinetic study of ozone-phenol reaction in aqueous solutions. AIChE J. 1979, 25, 583–591. [Google Scholar] [CrossRef]
- Lerner, H.-W.; Margraf, G.; Kretz, T.; Schiemann, O.; Bats, J.; Dürner, G.; Fabrizi de Biani, F.; Zanello, P.; Bolte, M.; Wagner, M. Redox behavior of pyrazolyl-substituted 1,4-dihydroxyarenes: Formation of the corresponding semiquinones, quinhydrones and quinones. Z. Naturforsch. B 2006, 61, 252–264. [Google Scholar] [CrossRef]
- Gupta, P.K. (Ed.) General toxicology. In Illustrated Toxicology: With Study Questions, 1st ed.; Academic Press: London, UK, 2018; pp. 1–65. [Google Scholar]
- de Furtado, R.X.S.; Sabatini, C.A.; Zaiat, M.; Azevedo, E.B. Perfluorooctane sulfonic acid (PFOS) degradation by optimized heterogeneous photocatalysis (TiO2/UV) using the response surface methodology (RSM). J. Water Process. Eng. 2021, 41, 101986. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Patience, G.S.; Boffito, D.C. Spray dried TiO2/WO3 heterostructure for photocatalytic applications with residual activity in the dark. Appl. Catal. B Environ. 2017, 226, 311–323. [Google Scholar] [CrossRef]
- Yiantzi, E.; Psillakis, E.; Tyrovola, K.; Kalogerakis, N. Vortex-assisted liquid–liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta 2010, 80, 2057–2062. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yu, L.; Wang, J.; Du, B.; Zhang, X. Enhanced catalytic activity of templated-double perovskite with 3D network structure for salicylic acid degradation under microwave irradiation: Insight into the catalytic mechanism. Chem. Eng. J. 2019, 368, 115–128. [Google Scholar] [CrossRef]









| Band | Wave Number (cm−1) | Attribution |
|---|---|---|
| I (a) | 1057 | stretching vibration of C–O bonds of alkoxy groups |
| II (a) | 1620 | stretching and bending vibration of OH groups |
| III (a) | 1726 | stretching vibration of C=O of carboxyl groups |
| IV (a) | 3320 | Stretching of the O–H bonds in hydroxyl groups |
| I (b) | 806 | breathing mode of the triazine units |
| II (b) | 1234; 1313; 1398 | stretching modes of C–N bond in heterocycles |
| III (b) | 1625 | stretching mode of C=N bonds |
| IV (b) | 3130 | stretching modes of secondary and primary amines |
| Scavenger | Removal (%) |
|---|---|
| None | 65.0 |
| IPA | 64.5 |
| BQ | 42.0 |
| KI | 60.0 |
| Scavenger | Inhibited Species |
|---|---|
| propan-2-ol (IPA) | hydroxyl radical (●OH) |
| benzoquin-1,4-one (BQ) | superoxide radical (O2●–) |
| potassium iodide (KI) | holes in the valence band (hBV+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, C.; Lopes, B.R.; Lima, C.d.S.; Ribeiro, C.; Azevedo, E.B. Using a Surface-Response Approach to Optimize the Photocatalytic Activity of rGO/g-C3N4 for Bisphenol A Degradation. Catalysts 2023, 13, 1069. https://doi.org/10.3390/catal13071069
Xavier C, Lopes BR, Lima CdS, Ribeiro C, Azevedo EB. Using a Surface-Response Approach to Optimize the Photocatalytic Activity of rGO/g-C3N4 for Bisphenol A Degradation. Catalysts. 2023; 13(7):1069. https://doi.org/10.3390/catal13071069
Chicago/Turabian StyleXavier, Chubraider, Bianca Rebelo Lopes, Cleyryson de Sousa Lima, Caue Ribeiro, and Eduardo Bessa Azevedo. 2023. "Using a Surface-Response Approach to Optimize the Photocatalytic Activity of rGO/g-C3N4 for Bisphenol A Degradation" Catalysts 13, no. 7: 1069. https://doi.org/10.3390/catal13071069
APA StyleXavier, C., Lopes, B. R., Lima, C. d. S., Ribeiro, C., & Azevedo, E. B. (2023). Using a Surface-Response Approach to Optimize the Photocatalytic Activity of rGO/g-C3N4 for Bisphenol A Degradation. Catalysts, 13(7), 1069. https://doi.org/10.3390/catal13071069