Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination
Abstract
:1. Introduction
2. Results
2.1. Microscopic Morphology and Structural Description
2.2. Photology and Electrochemistry Peculiarity
2.3. Photocatalytic Performance Evaluation
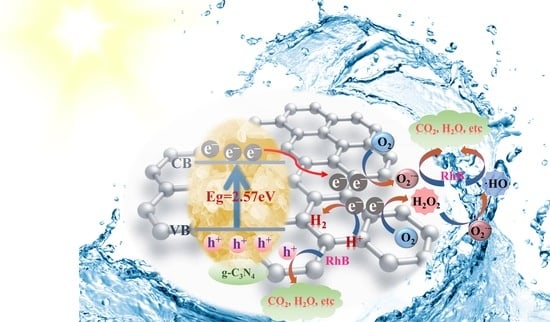
2.4. Mechanism Analysis of Photocatalytic Performance
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Catalysts
3.3. Photocatalytic Performance Valuation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, W.; Luo, J.-x.; Gu, Q.; Liu, Z.-H. Ultrathin 2D ZnGa-borate-LDH nanosheets for boosting dye-sensitized photocatalytic coupled reaction of H2 production with pollutant degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130575. [Google Scholar] [CrossRef]
- Mokhtar, M.; Shawky, A. Enhanced visible-light-driven H2 evolution over sol-gel prepared Nd2O3 supported with PtO nanoparticles. Ceram. Int. 2022, 48, 36670–36677. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Jin, Z. An orderly assembled g-C3N4, rGO and Ni2P photocatalyst for efficient hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 10316–10327. [Google Scholar] [CrossRef]
- Yang, H.; Li, E.; Zhou, B.; Wang, Y.; Li, P.; Xia, S. Preparation and characterization of a g-C3N4/LSACF composite and application in RhB degradation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1463–1472. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, D.; Zhou, H.; Pi, M.; Wang, X.; Chen, S. Coupling P Nanostructures with P-doped g-C3N4 as efficient visible light photocatalysts for H2 evolution and RhB degradation. ACS Sustain. Chem. Eng. 2018, 6, 6342–6349. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Luo, J.; Dai, Z.; Feng, M.; Gu, M.; Xie, Y. Graphitic carbon nitride/ferroferric oxide/reduced graphene oxide nanocomposite as highly active visible light photocatalyst. Nano Res. 2023, 16, 371–376. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Y.; Mei, J.; Li, Z.; Xu, S.; Yao, C. Synthesis of CdS/BiOBr nanosheets composites with efficient visible-light photocatalytic activity. J. Phys. Chem. Solids 2018, 112, 80–87. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, G.; Wei, J.; Guo, Z.; Wang, W.; Liu, X.; Song, Y. Activated carbon enhanced traditional activated sludge process for chemical explosion accident wastewater treatment. Environ. Res. 2023, 225, 115595. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Adhikari, S.; Mallya, D.S.; Thomas, M.; Surapaneni, A.; Moon, E.M.; Milne, N.A. Reuse of aluminium-based water treatment sludge for phosphorus adsorption: Evaluating the factors affecting and correlation between adsorption and sludge properties. Environ. Technol. Innov. 2022, 27, 102717. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zhu, C.; Chen, F.; Yang, Y.; Chen, X. Liberation and recovery of Cr from real tannery sludge by ultrasound-assisted supercritical water oxidation treatment. J. Clean. Prod. 2020, 267, 122064. [Google Scholar] [CrossRef]
- Hasan, I.; Albaeejan, M.A.; Alshayiqi, A.A.; Al-Nafaei, W.S.; Alharthi, F.A. In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange. Molecules 2023, 28, 1140. [Google Scholar] [PubMed]
- Bassi, A.; Qanungo, K.; Hasan, I.; Alshayiqi, A.A.; Ababtain, A.S.; Alharthi, F.A. CuO Nanorods Immobilized Agar-Alginate Biopolymer: A Green Functional Material for Photocatalytic Degradation of Amaranth Dye. Polymers 2023, 15, 553. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, M.; Fu, H.; Ruan, Z.; Sun, T.; Zhu, Y.; Wang, L.; Wang, Y.; Zhang, S. Synthesis of ultrathin porous g-C3N4 nanofilm via template-free method for photocatalytic degradation of tetracycline. J. Alloys Compd. 2023, 939, 168738. [Google Scholar] [CrossRef]
- Chen, J.; Shan, M.; Zhu, H.; Zhang, S.; Li, J.; Li, L. Antimicrobial properties of heterojunction BiSnSbO6-ZnO composites in wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 55498–55512. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Lin, B.; Chen, Z.; Xu, X.; Pan, M. Ti3C2TX MXene supported ZnO nanocomposites with highly efficient photocatalytic performance for degradation of VOCs. Diamond Relat. Mater. 2023, 133, 109763. [Google Scholar] [CrossRef]
- Sheng, J.; Yan, B.; He, B.; Lu, W.-D.; Li, W.-C.; Lu, A.-H. Nonmetallic boron nitride embedded graphitic carbon catalyst for oxidative dehydrogenation of ethylbenzene. Catal. Sci. Technol. 2020, 10, 1809–1815. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Yang, L.; Zhang, M.; Zhu, H.; Wang, F.; Yin, J. Co3O4 imbedded g-C3N4 heterojunction photocatalysts for visible-light-driven hydrogen evolution. Renew. Energ. 2020, 145, 691–698. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Vijayarajan, V.S.; Rishi Krishna, B.S.; Veluswamy, P.; Neppolian, B. Cobalt phosphate hydroxide loaded g-C3N4 photocatalysts and its hydrogen production activity. Int. J. Hydrogen Energy 2020, 45, 7562–7573. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Zhao, Q.; Niu, T.; Pan, L.; Xu, P.; Zhang, F.; Wu, W.; Ni, T. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar] [CrossRef]
- Wang, J.; Gao, B.; Dou, M.; Huang, X.; Ma, Z. A porous g-C3N4 nanosheets containing nitrogen defects for enhanced photocatalytic removal meropenem: Mechanism, degradation pathway and DFT calculation. Environ. Res. 2020, 184, 109339. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Zhang, H.; Yang, Z.; Zhou, L.; Pan, L.; Li, C.; Yang, Z.; Liu, D. Enhanced adsorption and catalytic degradation of antibiotics by porous 0D/3D Co3O4/g-C3N4 activated peroxymonosulfate: An experimental and mechanistic study. J. Colloid Interf. Sci. 2022, 625, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-F.; Chen, J.-J.; Huang, Y.-X.; Zhang, X.; Wang, W.-K.; Huang, G.-X.; Yu, H.-Q. Selective electrochemical CO2 reduction on Cu-Pd heterostructure. Appl. Catal. B Environ. 2020, 270, 118864. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, P.; Hu, S.; Wu, X.; He, J.; Wu, Z.; Wang, W.; Zheng, P.; Zheng, H.; Zheng, L.; et al. Broadband photodetector based on ReS2/graphene/WSe2 heterostructure. Nanotechnology 2021, 32, 465201. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.-P.; Zhao, J.-X.; Ge, Z.-H.; Zhao, X.-K.; Zou, L. ZnO/carbon quantum dots heterostructure with enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 279, 367–373. [Google Scholar] [CrossRef]
- Vidyasagar, D.; Kumari, V.; Bhoyar, T.; Umare, S.S. Unveiling morphology altered photoactivity of microspherical carbon nitride scaffolds. Appl. Surf. Sci. 2020, 526, 146661. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, Z.; Liu, Y.; Shi, C.; Zhu, M.; Wang, A. Construction of 2D/2D BiVO4/g-C3N4 nanosheet heterostructures with improved photocatalytic activity. J. Colloid Interf. Sci. 2019, 533, 251–258. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Zhang, M.; Li, Q.; Yang, J. Construction of 2D/2D TiO2/g-C3N4 nanosheet heterostructures with improved photocatalytic activity. Mater. Res. Bull. 2020, 125, 110765. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.; Dai, Y.; Ma, C.; Yang, G. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Jia, J.; Sun, W.; Zhang, Q.; Zhang, X.; Hu, X.; Liu, E.; Fan, J. Inter-plane heterojunctions within 2D/2D FeSe2/g-C3N4 nanosheet semiconductors for photocatalytic hydrogen generation. Appl. Catal. B Environ. 2020, 261, 118249. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Xia, Y.; Liang, R.; Yang, M.-Q.; Zhu, S.; Yan, G. Construction of chemically bonded interface of organic/inorganic g-C3N4/LDH heterojunction for Z-schematic photocatalytic H2 generation. Nanomaterials 2021, 11, 2762. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Xu, D.; Liu, Q.; Tang, H. Construction of g-C3N4 nanotube/Ag3PO4 S-scheme heterojunction for enhanced photocatalytic oxygen generation. Ceram. Int. 2022, 48, 2169–2176. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, B.; Asakura, Y.; Tsukuda, S.; Kato, H.; Kakihana, M.; Yin, S. Alkali-assisted hydrothermal preparation of g-C3N4/rGO nanocomposites with highly enhanced photocatalytic NOx removal activity. Appl. Surf. Sci. 2020, 521, 146213. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, D.; Chen, Q.; Chao, C.; Sun, J.; Dong, S.; Sun, Y. Synthesis of rGO/g-C3N4 for methyl orange degradation in activating peroxydisulfate under simulated solar light irradiation. J. Alloys Compd. 2022, 907, 164500. [Google Scholar] [CrossRef]
- Palanivel, B.; Lallimathi, M.; Arjunkumar, B.; Shkir, M.; Alshahrani, T.; Al-Namshah, K.S.; Hamdy, M.S.; Shanavas, S.; Venkatachalam, M.; Ramalingam, G. RGO supported g-C3N4/CoFe2O4 heterojunction: Visible-light-active photocatalyst for effective utilization of H2O2 to organic pollutant degradation and OH radicals production. J. Environ. Chem. Eng. 2021, 9, 104698. [Google Scholar] [CrossRef]
- Xiao, P.; Jiang, D.; Ju, L.; Jing, J.; Chen, M. Construction of RGO/CdIn2S4/g-C3N4 ternary hybrid with enhanced photocatalytic activity for the degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2018, 433, 388–397. [Google Scholar] [CrossRef]
- Fu, R.; Yu, P.; Wang, M.; Sun, J.; Chen, D.; Jin, C.; Li, Z. The research of lead ion detection based on rGO/g-C3N4 modified glassy carbon electrode. Microchem. J. 2020, 157, 105076. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Dye degradation studies using immobilized pristine and waste polystyrene-TiO2/rGO/g-C3N4 nanocomposite photocatalytic film in a novel airlift reactor under solar light. J. Environ. Chem. Eng. 2019, 7, 103289. [Google Scholar] [CrossRef]
- Swetha, S.; Abdel-Maksoud, M.A.; Okla, M.K.; Janani, B.; Dawoud, T.M.; El-Tayeb, M.A.; Sudheer Khan, S. Triple-mechanism driven Fe-doped n-n hetero-architecture of Pr6O11-MoO3 decorated g-C3N4 for doxycycline degradation and bacterial photoinactivation. Chem. Eng. J. 2023, 461, 141806. [Google Scholar] [CrossRef]
- Palanivel, B.; Hossain, M.S.; Raghu, M.S.; Kumar, K.Y.; Macadangdang, R.R.; Ubaidullah, M.; Prakash, C.; Bommireddy, P.R.; Park, S.-H. Green synthesis of Fe2O3 deposited g-C3N4: Addition of rGO promoted Z-scheme ternary heterojunction for efficient photocatalytic degradation and H2 evolution reaction. Mater. Res. Bull. 2023, 162, 112177. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, H.; Li, D.; Xu, F. Construction of Fe3S4/g-C3N4 composites as photo-Fenton-like catalysts to realize high-efficiency degradation of pollutants. Ceram. Int. 2023, 49, 16070–16079. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. A facile fabrication of Br-modified g-C3N4/rGO composite catalyst for enhanced visible photocatalytic activity towards the degradation of harmful dyes. Mater. Res. Bull. 2020, 130, 110870. [Google Scholar] [CrossRef]
- Ali, G.; Jazib Abbas Zaidi, S.; Abdul Basit, M.; Park, T.J. Synergetic performance of systematically designed g-C3N4/rGO/SnO2 nanocomposite for photodegradation of Rhodamine-B dye. Appl. Surf. Sci. 2021, 570, 151140. [Google Scholar] [CrossRef]
- Lökçü, E.; Kaçar, N.; Çayirli, M.; Özden, R.C.; Anik, M. Photoassisted charging of li-ion oxygen batteries using g-C3N4/rGO nanocomposite photocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 34583–34592. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Li, J.; Wang, C.; Pan, L.; Wang, G. Synergistic coupling of NiS1.03 nanoparticle with S-doped reduced graphene oxide for enhanced lithium and sodium storage. Chem. Eng. J. 2021, 407, 127199. [Google Scholar] [CrossRef]
- Yu, C.; Hou, J.; Zhang, B.; Liu, S.; Pan, X.; Song, H.; Hou, X.; Yan, Q.; Zhou, C.; Liu, G.; et al. In-situ electrodeposition synthesis of Z-scheme rGO/g-C3N4/TNAs photoelectrodes and its degradation mechanism for oxytetracycline in dual-chamber photoelectrocatalytic system. J. Environ. Manag. 2022, 308, 114615. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, S.; Cao, C.; Hong, E.; Zeng, L.; Yang, W.; Huang, L.; Yang, C. Enhanced light harvesting and charge separation of carbon and oxygen co-doped carbon nitride as excellent photocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 612, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, H.Y.; Lakhera, S.K.; Shankar, M.V.; Neppolian, B. Synergetic improvement in charge carrier transport and light harvesting over ternary InVO4-g-C3N4/rGO hybrid nanocomposite for hydrogen evolution reaction. Int. J. Hydrogen Energy. 2020, 45, 7530–7540. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, S.; Wang, X.; Huo, S.; Lu, J.; Ma, B.; Ma, X.; Liu, W.; Gao, M.; Xie, H. Photoelectrocatalytic degradation of m-chloronitrobenzene through rGO/g-C3N4/TiO2 nanotube arrays photoelectrode under visible light: Performance, DFT calculation and mechanism. Sep. Purif. Technol. 2022, 302, 121944. [Google Scholar] [CrossRef]
- Li, X.; Shen, D.; Liu, C.; Li, J.; Zhou, Y.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Fabricated rGO-modified Ag2S nanoparticles/g-C3N4 nanosheets photocatalyst for enhancing photocatalytic activity. J. Colloid Interface Sci. 2019, 554, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, J.; Qian, F.; Min, Y. Magnetic BiFeO3 grafted with MWCNT hybrids as advanced photocatalysts for removing organic contamination with a high concentration. RSC Adv. 2016, 6, 49966–49972. [Google Scholar] [CrossRef]
- Karimi-Harandi, M.-H.; Shabani-Nooshabadi, M.; Darabi, R. Simultaneous determination of citalopram and selegiline using an efficient electrochemical sensor based on ZIF-8 decorated with RGO and g-C3N4 in real samples. Anal. Chim. Acta 2022, 1203, 339662. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, K.; Bavani, T.; Arunachalam, P.; Lee, S.J.; Theerthagiri, J.; Madhavan, J.; Pollet, B.G.; Choi, M.Y. Nanofiber NiMoO4/g-C3N4 composite electrode materials for redox supercapacitor applications. Nanomaterials 2020, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. Fabrication of metal-free 2D/2D g-C3N4/rGO composite towards the degradation of harmful organics. Optik 2020, 219, 165023. [Google Scholar] [CrossRef]
- Lu, N.; Wang, P.; Su, Y.; Yu, H.; Liu, N.; Quan, X. Construction of Z-Scheme g-C3N4/RGO/WO3 with in situ photoreduced graphene oxide as electron mediator for efficient photocatalytic degradation of ciprofloxacin. Chemosphere 2019, 215, 444–453. [Google Scholar] [CrossRef]
- Ren, S.; Dong, J.; Duan, X.; Cao, T.; Yu, H.; Lu, Y.; Zhou, D. A novel (Zr/Ce)UiO-66(NH2)@g-C3N4 Z-scheme heterojunction for boosted tetracycline photodegradation via effective electron transfer. Chem. Eng. J. 2023, 460, 141884. [Google Scholar] [CrossRef]
- Li, C.; Kan, C.; Meng, X.; Liu, M.; Shang, Q.; Yang, Y.; Wang, Y.; Cui, X. Self-assembly 2D Ti3C2/g-C3N4 MXene heterojunction for highly efficient photocatalytic degradation of tetracycline in visible wavelength range. Nanomaterials 2022, 12, 4015. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Feng, Y.; Xie, Z.; Zhang, Q.; Jin, X.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. Facile synthesis of carbon quantum dots loaded with mesoporous g-C3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics. Dalton Trans. 2018, 47, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Shafiq, M.; Hamid, S.; Shafiee, A.; Usman, M.; Khan, I.; Ashiq, M.N.; Arfan, M. Reactive oxygen species: New insights into photocatalytic pollutant degradation over g-C3N4/ZnSe nanocomposite. Appl. Surf. Sci. 2020, 532, 147418. [Google Scholar] [CrossRef]
- Choi, H.; Surendran, S.; Sim, Y.; Je, M.; Janani, G.; Choi, H.; Kim, J.K.; Sim, U. Enhanced electrocatalytic full water-splitting reaction by interfacial electric field in 2D/2D heterojunction. Chem. Eng. J. 2022, 450, 137789. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Vidyasagar, D.; Bak, N.-h.; Jung, N.; Kim, Y.-H.; Lee, J.-H.; Kim, S.-G.; Kim, M.-D. UV light driven high-performance room temperature surface acoustic wave NH3 gas sensor using sulfur-doped g-C3N4 quantum dots. Nano Res. 2023, 16, 7682–7695. [Google Scholar] [CrossRef]
- Gupta, A.; Bhoyar, T.; Abraham, B.M.; Kim, D.J.; Pasupuleti, K.S.; Umare, S.S.; Vidyasagar, D.; Gedanken, A. Potassium molten salt-mediated in situ structural reconstruction of a carbon nitride photocatalyst. ACS Appl. Mater. Interfaces 2023, 15, 18898–18906. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, C.; Cao, M.; Wang, B.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Defects controlling, elements doping, and crystallinity improving triplestrategy modified carbon nitride for efficient photocatalytic diclofenac degradation and H2O2 production. Appl. Catal. B Environ. 2023, 321, 121941. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Z.; Li, Y.; Jia, P.; Liu, T. Study on photocatalytic activity of Ag2O modified BiOI/g-C3N4 composite photocatalyst for degradation of RhB. J. Electron. Mater. 2022, 51, 5508–5520. [Google Scholar] [CrossRef]
- Ji, S.; Yang, Y.; Zhou, Z.; Li, X.; Liu, Y. Photocatalysis-Fenton of Fe-doped g-C3N4 catalyst and its excellent degradation performance towards RhB. J. Water Process. Eng. 2021, 40, 101804. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, Z.; Ma, M.; Li, P.; Lu, J.; Hou, Y.; Chen, X.; Duo, S. Enhanced visible-light photocatalytic performance of SAPO-5-based g-C3N4 composite for Rhodamine B (RhB) degradation. Materials 2019, 12, 3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Sun, H.; Li, C.; Wang, M.; Wu, J.; Chen, M.; Jiang, S.; Niu, T.; Liu, D. Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination. Catalysts 2023, 13, 1079. https://doi.org/10.3390/catal13071079
Zhao C, Sun H, Li C, Wang M, Wu J, Chen M, Jiang S, Niu T, Liu D. Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination. Catalysts. 2023; 13(7):1079. https://doi.org/10.3390/catal13071079
Chicago/Turabian StyleZhao, Congyue, Hengchao Sun, Chunling Li, Manrong Wang, Jiahang Wu, Minghui Chen, Shuai Jiang, Tianqi Niu, and Dong Liu. 2023. "Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination" Catalysts 13, no. 7: 1079. https://doi.org/10.3390/catal13071079
APA StyleZhao, C., Sun, H., Li, C., Wang, M., Wu, J., Chen, M., Jiang, S., Niu, T., & Liu, D. (2023). Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination. Catalysts, 13(7), 1079. https://doi.org/10.3390/catal13071079