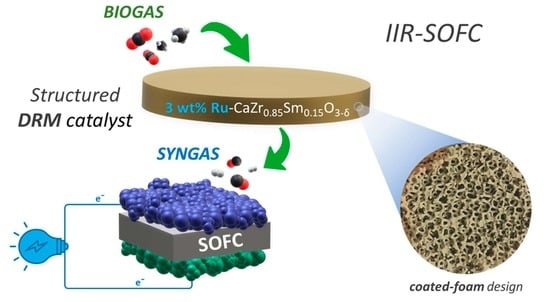
Structured Catalyst for Indirect Internal Reforming (IIR) of Biogas in Solid Oxide Fuel Cell (SOFC)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ru3CZS Powder
2.2. Structured Catalysts
2.3. Temperature–Programmed Reduction (TPR)
2.4. Catalytic Activity in Biogas
2.5. Spent Catalysts
2.6. Cell Test in IIR–Mode
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Structured Catalyst Fabrication
3.3. Structured Catalyst Characterization
3.4. Catalytic Tests Apparatus
3.5. IIR–SOFC Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duerr, M.; Gair, S.; Cruden, A.; McDonald, J. Hydrogen and electrical energy from organic waste treatment. Int. J. Hydrogen Energy 2007, 32, 705–709. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.-V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri–Reforming of Methane. In Hydrogen Supply Chain: Design, Deployment and Operation; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–166. [Google Scholar] [CrossRef] [Green Version]
- Mizera, A.; Błaszczak, P.; Bochentyn, B.; Lach, R.; Drożdż, E. Cu supported on various oxides as a candidate catalyst for dry methane reforming in DIR–SOFCs systems. Int. J. Hydrogen Energy 2022, 47, 25647–25661. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry–reforming of methane over bimetallic Ni–M/La2O3 (M=Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni–Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2016, 281, 250–258. [Google Scholar] [CrossRef]
- Turap, Y.; Wang, I.; Fu, T.; Wu, Y.; Wang, Y. Co–Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 6538–6548. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Pintar, A. Biogas to syngas conversion without carbonaceous deposits via the dry reforming reaction using transition metal catalysts. Catal. Today 2015, 253, 155–162. [Google Scholar] [CrossRef]
- Majewski, A.J.; Wood, J.; Bujalski, W. Nickel–silica core@shell catalyst for methane reforming. Int. J. Hydrogen Energy 2013, 38, 14531–14541. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Begum, N.; Ito, S.-I.; Tomishige, K. Oxidative steam reforming of methane over Ni/α–Al2O3 modified with trace noble metals. Appl. Catal. A Gen. 2009, 358, 186–192. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto–Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Erdohelyi, A.; Cserenyi, J.; Solymosi, F. Activation of CH4 and Its Reaction with CO2 over Supported Rh Catalysts. J. Catal. 1993, 141, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Gurav, H.R.; Dama, S.; Samuel, V.; Chilukuri, S. Influence of preparation method on activity and stability of Ni catalysts supported on Gd doped ceria in dry reforming of methane. J. CO2 Util. 2017, 20, 357–367. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Lin, S.; Luo, Y.; Bao, Z.; Mao, Y.; Li, K.; Wu, D.; Peng, H. Confined Ni–In intermetallic alloy nanocatalyst with excellent coking resistance for methane dry reforming. J. Energy Chem. 2021, 65, 34–47. [Google Scholar] [CrossRef]
- Ahn, S.; Littlewood, P.; Liu, Y.; Marks, T.J.; Stair, P.C. Stabilizing Supported Ni Catalysts for Dry Reforming of Methane by Combined La Doping and Al Overcoating Using Atomic Layer Deposition. ACS Catal. 2022, 12, 10522–10530. [Google Scholar] [CrossRef]
- Mortazavi-Manesh, A.; Safari, N.; Bahadoran, F.; Khani, Y. Synthesis, characterization, and methanol steam reforming performance of Cu/perovskite–structured catalysts. Heliyon 2023, 9, e13742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Duranti, L.; Sora, I.N.; Zurlo, F.; Luisetto, I.; Licoccia, S.; Di Bartolomeo, E. The role of manganese substitution on the redox behavior of La0.6Sr0.4Fe0.8Mn0.2O3−δ. J. Eur. Ceram. Soc. 2020, 40, 4076–4083. [Google Scholar] [CrossRef]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry reforming of methane using various catalysts in the process. Biomass Convers. Biorefin. 2020, 10, 567–587. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Fundamentals and Applications of Structured Ceramic Foam Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4166–4177. [Google Scholar] [CrossRef]
- Santoro, M.; Luisetto, I.; Tuti, S.; Licoccia, S.; Romano, C.; Notargiacomo, A.; Di Bartolomeo, E. Nickel–Based Structured Catalysts for Indirect Internal Reforming of Methane. Appl. Sci. 2020, 10, 3083. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Ge, B.-H.; Liu, T.-F.; Yang, Y.-Z.; Li, B.; Li, W.-Z. Robust Ruthenium–Saving Catalyst for High–Temperature Carbon Dioxide Reforming of Methane. ACS Catal. 2019, 10, 783–791. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Ashok, J.; Kawi, S. A comprehensive review of anti–coking, anti–poisoning and anti–sintering catalysts for biomass tar reforming reaction. Chem. Eng. Sci. X 2020, 7, 100065. [Google Scholar] [CrossRef]
- Shiratori, Y.; Ijichi, T.; Oshima, T.; Sasaki, K. Internal reforming SOFC running on biogas. Int. J. Hydrogen Energy 2010, 35, 7905–7912. [Google Scholar] [CrossRef]
- Chiodo, V.; Galvagno, A.; Lanzini, A.; Papurello, D.; Urbani, F.; Santarelli, M.; Freni, S. Biogas reforming process investigation for SOFC application. Energy Convers. Manag. 2015, 98, 252–258. [Google Scholar] [CrossRef]
- Faro, M.L.; Antonucci, V.; Antonucci, P.; Aricò, A. Fuel flexibility: A key challenge for SOFC technology. Fuel 2012, 102, 554–559. [Google Scholar] [CrossRef]
- Sarno, C.; Luisetto, I.; Zurlo, F.; Licoccia, S.; Di Bartolomeo, E. Lanthanum chromite based composite anodes for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 14742–14750. [Google Scholar] [CrossRef]
- Santoro, M.; Di Bartolomeo, E.; Luisetto, I.; Aricò, A.; Squadrito, G.; Zignani, S.; Faro, M.L. Insights on the electrochemical performance of indirect internal reforming of biogas into a solid oxide fuel cell. Electrochim. Acta 2022, 409, 139940. [Google Scholar] [CrossRef]
- Luisetto, I.; Mancini, M.R.; Della Seta, L.; Chierchia, R.; Vanga, G.; Grilli, M.L.; Stendardo, S. CaO–CaZrO3 Mixed Oxides Prepared by Auto–Combustion for High Temperature CO2 Capture: The Effect of CaO Content on Cycle Stability. Metals 2020, 10, 750. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Lawson, S.M.; Greaves, G.; Blackburn, L.R.; Chapman, R.; Hyatt, N.C.; Corkhill, C.L. Synthesis and in situ ion irradiation of A–site deficient zirconate perovskite ceramics. J. Mater. Chem. A 2020, 8, 19454–19466. [Google Scholar] [CrossRef]
- Kim, D.H.; Yu, B.Y.; Cha, P.R.; Yoon, W.Y.; Byun, J.Y.; Kim, S.H. A study on FeCrAl foam as effective catalyst support under thermal and mechanical stresses. Surf. Coat. Technol. 2012, 209, 169–176. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; AlKhoori, A.; Gaber, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Towards maximizing conversion of ethane and carbon dioxide into synthesis gas using highly stable Ni–perovskite catalysts. J. CO2 Util. 2022, 61, 102046. [Google Scholar] [CrossRef]
- Madhavaram, H.; Idriss, H.; Wendt, S.; Kim, Y.; Knapp, M.; Over, H.; Aßmann, J.; Löffler, E.; Muhler, M. Oxidation Reactions over RuO2: A Comparative Study of the Reactivity of the (110) Single Crystal and Polycrystalline Surfaces. J. Catal. 2001, 202, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Roine, A. HSC–software Ver. 3.0 for thermodynamic calculations. In Proceedings of the International Symposium on Computer Software in Chemical and Extractive Metallurgy; Elsevier: Amsterdam, The Netherlands, 1989; pp. 15–29. [Google Scholar]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Seo, M.; Kim, S.Y.; Kim, Y.D.; Park, E.D.; Uhm, S. Highly stable barium zirconate supported nickel oxide catalyst for dry reforming of methane: From powders toward shaped catalysts. Int. J. Hydrogen Energy 2018, 43, 11355–11362. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Khairudin, N.F.; Mohammadi, M.; Mohamed, A.R. An investigation on the relationship between physicochemical characteristics of alumina–supported cobalt catalyst and its performance in dry reforming of methane. Environ. Sci. Pollut. Res. 2021, 28, 29157–29176. [Google Scholar] [CrossRef]
- Dreyer, J.A.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.H.-L.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru–based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2019, 356, 419–432. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Nijdam, T.; Jeurgens, L.; Sloof, W. Promoting exclusive α–Al2O3 growth upon high–temperature oxidation of NiCrAl alloys: Experiment versus model predictions. Acta Mater. 2005, 53, 1643–1653. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. Highly Stable and Efficient Catalyst with In Situ Exsolved Fe–Ni Alloy Nanospheres Socketed on an Oxygen Deficient Perovskite for Direct CO2 Electrolysis. ACS Catal. 2016, 6, 6219–6228. [Google Scholar] [CrossRef]
- Marasi, M.; Panunzi, A.P.; Duranti, L.; Lisi, N.; Di Bartolomeo, E. Enhancing Oxygen Reduction Activity and Structural Stability of La0.6Sr0.4FeO3−δ by 1 mol % Pt and Ru B–Site Doping for Application in All–Perovskite IT–SOFCs. ACS Appl. Energy Mater. 2022, 5, 2918–2928. [Google Scholar] [CrossRef]
- Marasi, M.; Duranti, L.; Luisetto, I.; Fabbri, E.; Licoccia, S.; Di Bartolomeo, E. Ru–doped lanthanum ferrite as a stable and versatile electrode for reversible symmetric solid oxide cells (r–SSOCs). J. Power Sources 2023, 555, 232399. [Google Scholar] [CrossRef]
- Duranti, L.; Luisetto, I.; Licoccia, S.; D’Ottavi, C.; di Bartolomeo, E. Novel Composite Fuel Electrode for CO2/CO–RSOCs. J. Electrochem. Soc. 2021, 168, 104507. [Google Scholar] [CrossRef]
- Duranti, L.; Luisetto, I.; Licoccia, S.; Del Gaudio, C.; Di Bartolomeo, E. Electrochemical performance and stability of LSFMn+NiSDC anode in dry methane. Electrochimica Acta 2020, 362, 137116. [Google Scholar] [CrossRef]
- Duranti, L.; Luisetto, I.; Casciardi, S.; Del Gaudio, C.; Di Bartolomeo, E. Multi–functional, high–performing fuel electrode for dry methane oxidation and CO2 electrolysis in reversible solid oxide cells. Electrochim. Acta 2021, 394, 139163. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J. Coupling interface constructions of NiO–Cr2O3 heterostructures for efficient electrocatalytic oxygen evolution. Electrochim. Acta 2019, 320, 134577. [Google Scholar] [CrossRef]













| Element | Nominal wt% | Measured wt% |
|---|---|---|
| O | 25.2 | 25.8 ± 2.4 |
| Ca | 20.4 | 20.2 ± 3.8 |
| Zr | 39.8 | 38. 4 ± 1.5 |
| Sm | 11.6 | 11.2 ± 0.9 |
| Ru | 3 | 3.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prioriello, A.; Duranti, L.; Luisetto, I.; Sanna, F.; Larosa, C.; Grilli, M.L.; Di Bartolomeo, E. Structured Catalyst for Indirect Internal Reforming (IIR) of Biogas in Solid Oxide Fuel Cell (SOFC). Catalysts 2023, 13, 1129. https://doi.org/10.3390/catal13071129
Prioriello A, Duranti L, Luisetto I, Sanna F, Larosa C, Grilli ML, Di Bartolomeo E. Structured Catalyst for Indirect Internal Reforming (IIR) of Biogas in Solid Oxide Fuel Cell (SOFC). Catalysts. 2023; 13(7):1129. https://doi.org/10.3390/catal13071129
Chicago/Turabian StylePrioriello, Anna, Leonardo Duranti, Igor Luisetto, Frederick Sanna, Claudio Larosa, Maria Luisa Grilli, and Elisabetta Di Bartolomeo. 2023. "Structured Catalyst for Indirect Internal Reforming (IIR) of Biogas in Solid Oxide Fuel Cell (SOFC)" Catalysts 13, no. 7: 1129. https://doi.org/10.3390/catal13071129
APA StylePrioriello, A., Duranti, L., Luisetto, I., Sanna, F., Larosa, C., Grilli, M. L., & Di Bartolomeo, E. (2023). Structured Catalyst for Indirect Internal Reforming (IIR) of Biogas in Solid Oxide Fuel Cell (SOFC). Catalysts, 13(7), 1129. https://doi.org/10.3390/catal13071129