A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Poor Electrocatalytic Performance of NixFeyOOH in Saline–Alkali Water (SAW)
2.2. Structure Characterization of NC/NixFeyOOH Catalyst
2.3. Electrocatalytic Performance of NC/NixFeyOOH Catalyst
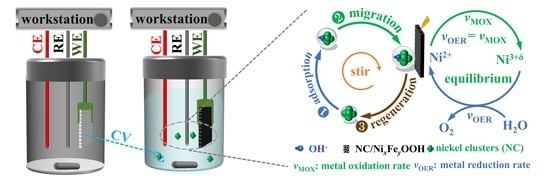
2.4. Possible Reasons for the Self-Healing NC/NixFeyOOH Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of NiFe-MOFs
3.3. Synthesis of Nickel Clusters (NC) and Iron Clusters and Nickel–Iron Clusters
3.4. Synthesis of NixFeyOOH and NC/NixFeyOOH
3.5. Characterizations
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shi, R.; Jiao, S.; Li, M.; Li, Q. Structural Design for Electrocatalytic Water Splitting to Realize Industrial-Scale Deployment: Strategies, Advances, and Perspectives. J. Energy Chem. 2022, 70, 129–153. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.J.; Li, Z.Y.; Bu, X.H. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, e2003916. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Luo, Y.; Yu, Q.; Zhang, Z.; Zhang, S.; Liu, Z.; Ren, W.; Cheng, H.M.; Li, J.; Liu, B. A Durable and Efficient Electrocatalyst for Saline Water Splitting with Current Density Exceeding 2000 mA cm−2. Adv. Funct. Mater. 2021, 31, 2010367. [Google Scholar] [CrossRef]
- Vorosmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of Low-Grade and Saline Surface Water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Liu, X.; Chi, J.; Mao, H.; Wang, L. Principles of Designing Electrocatalyst to Boost Reactivity for Seawater Splitting. Adv. Energy Mater. 2023, 13, 2301438. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct Seawater Electrolysis by Adjusting the Local Reaction Environment of a Catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Bigiani, L.; Barreca, D.; Gasparotto, A.; Andreu, T.; Verbeeck, J.; Sada, C.; Modin, E.; Lebedev, O.I.; Morante, J.R.; Maccato, C. Selective Anodes for Seawater Splitting Via Functionalization of Manganese Oxides by a Plasma-Assisted Process. Appl. Catal. B Environ. 2021, 284, 119684. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A Membrane-Based Seawater Electrolyser for Hydrogen Generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Nocera, D.G. Self-Healing Catalysis in Water. Proc. Natl. Acad. Sci. USA 2017, 114, 13380–13384. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Veroneau, S.S.; Nocera, D.G. Self-Healing Oxygen Evolution Catalysts. Nat. Commun. 2022, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, F.; Liu, Z.; Nakabayashi, M.; Xiao, Y.; Zeng, Q.; Fu, J.; Wu, Q.; Cui, C.; Han, Y.; et al. A Self-Healing Catalyst for Electrocatalytic and Photoelectrochemical Oxygen Evolution in Highly Alkaline Conditions. Nat. Commun. 2021, 12, 5980. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Lopes, P.P.; Farinazzo Bergamo Dias Martins, P.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic Stability of Active Sites in Hydr(Oxy)Oxides for the Oxygen Evolution Reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, F.; Qin, C.; Wu, S.; Cao, M.; Yang, P.; Huang, L.; Wu, Y. A Self-Healing Electrocatalytic System Via Electrohydrodynamics Induced Evolution in Liquid Metal. Nat. Commun. 2022, 13, 7625. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tu, W.; Mu, L.; Sun, Z.; Hu, Q.; Yang, Y. Saline Alkali Water Desalination Project in Southern Xinjiang of China: A Review of Desalination Planning, Desalination Schemes and Economic Analysis. Renew. Sustain. Energy Rev. 2019, 113, 109268. [Google Scholar] [CrossRef]
- Thangavel, P.; Ha, M.; Kumaraguru, S.; Meena, A.; Singh, A.N.; Harzandi, A.M.; Kim, K.S. Graphene-Nanoplatelets-Supported NiFe-MOF: High-Efficiency and Ultra-Stable Oxygen Electrodes for Sustained Alkaline Anion Exchange Membrane Water Electrolysis. Energy Environ. Sci. 2020, 13, 3447–3458. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Dastafkan, K.; Zhao, C.; Zhao, X.; Xue, Y.; Huo, J.; Li, S.; Zhai, Q. Lattice Matching Growth of Conductive Hierarchical Porous MOF/LDH Heteronanotube Arrays for Highly Efficient Water Oxidation. Adv. Mater. 2021, 33, e2006351. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; Xu, S.; Duan, J.; Chen, S. Metallic Two-Dimensional Metal-Organic Framework Arrays for Ultrafast Water Splitting. J. Power Sources 2021, 494, 229733. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; Ortiz-Ledon, C.A.; Zhu, J.; Chen, S.; Duan, J. Biomimetic Assembly to Superplastic Metal-Organic Framework Aerogels for Hydrogen Evolution from Seawater Electrolysis. Exploration 2021, 1, 20210021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, W.; Wang, M.; Xi, S.; Meng, J.; Zhao, K.; Jin, J.; Xu, W.; Wang, Z.; Liu, X.; et al. Low-Crystalline Bimetallic Metal-Organic Framework Electrocatalysts with Rich Active Sites for Oxygen Evolution. ACS Energy Lett. 2018, 4, 285–292. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal-Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800584. [Google Scholar] [CrossRef]
- Marques, L.F.; Santos, H.P.; Correa, C.C.; Resende, J.A.L.C.; da Silva, R.R.; Ribeiro, S.J.L.; Machado, F.C. Construction of a Series of Rare Earth Metal-Organic Frameworks Supported by Thiophenedicarboxylate Linker: Synthesis, Characterization, Crystal Structures and near-Infrared/Visible Luminescence. Inorg. Chim. Acta 2016, 451, 41–51. [Google Scholar] [CrossRef]
- Wang, G.X.; Shang, L.L.; Li, Z.H.; Zhao, B.T. A New Two-Dimensional Manganese(II) Coordination Polymer Based on Thiophene-3,4-Dicarboxylic Acid. Acta Crystallogr. C 2014, 70, 715–717. [Google Scholar] [CrossRef]
- Fei, H.; Liu, X.; Li, Z.; Feng, W. Synthesis of Manganese Coordination Polymer Microspheres for Lithium-Ion Batteries with Good Cycling Performance. Electrochim. Acta 2015, 174, 1088–1095. [Google Scholar] [CrossRef]
- Luo, R.; Li, Y.; Xing, L.; Wang, N.; Zhong, R.; Qian, Z.; Du, C.; Yin, G.; Wang, Y.; Du, L. A Dynamic Ni(OH)2-NiOOH/NiFeP Heterojunction Enabling High-Performance E-Upgrading of Hydroxymethylfurfural. Appl. Catal. B Environ. 2022, 311, 121357. [Google Scholar] [CrossRef]
- Wu, B.; Gong, S.; Lin, Y.; Li, T.; Chen, A.; Zhao, M.; Zhang, Q.; Chen, L. A Unique NiOOH@FeOOH Heteroarchitecture for Enhanced Oxygen Evolution in Saline Water. Adv. Mater. 2022, 34, e2108619. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Zhai, P.; Wang, C.; Gao, J.; Sun, L.; Hou, J. Triggering Lattice Oxygen Activation of Single-Atomic Mo Sites Anchored on Ni-Fe Oxyhydroxides Nanoarrays for Electrochemical Water Oxidation. Adv. Mater. 2022, 34, e2202523. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Z.; Huang, X.; Qin, Y.; Yang, H.; Ren, X.; Zhang, Q.; Liu, J.; Shao, M.; He, C. Integrating Well-Controlled Core-Shell Structures into “Superaerophobic” Electrodes for Water Oxidation at Large Current Densities. Appl. Catal. B Environ. 2021, 286, 119920. [Google Scholar] [CrossRef]
- Huang, L.F.; Hutchison, M.J.; Santucci, R.J.; Scully, J.R.; Rondinelli, J.M. Improved Electrochemical Phase Diagrams from Theory and Experiment: The Ni-Water System and Its Complex Compounds. J. Phys. Chem. C 2017, 121, 9782–9789. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Duan, J.; Chen, S. Porous yet Densely Packed Metal-Organic Frameworks (MOFs) toward Ultrastable Oxygen Evolution at Practical Current Densities. Mater. Chem. Front. 2023, 7, 5005–5014. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Yang, F.; Lopez Luna, M.; Haase, F.T.; Escalera-Lopez, D.; Yoon, A.; Ruscher, M.; Rettenmaier, C.; Jeon, H.S.; Ortega, E.; Timoshenko, J.; et al. Spatially and Chemically Resolved Visualization of Fe Incorporation into NiO Octahedra During the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 21465–21474. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, W.; Guo, S.; Pan, J.; Hu, J.; Xu, X. Iron-Locked Hydr(Oxy)Oxide Catalysts Via Ion-Compensatory Reconstruction Boost Large-Current-Density Water Oxidation. Adv. Sci. 2023, 10, e2300717. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhu, Q.; Shi, L.; Wang, F.; Ma, J.; Sun, J. The Efficient Removal of Dyes over Magnetic Ni Embedded on Mesoporous Graphitic Carbon Material. J. Porous Mater. 2014, 21, 985–991. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, D.; Chen, J.; Tian, J.; Isimjan, T.T.; Yang, X. Oxygen-Evolution Catalysts Based on Iron-Mediated Nickel Metal-Organic Frameworks. ACS Appl. Nano Mater. 2019, 2, 6334–6342. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Kim, D.; Liu, M.; Lee, L.Y.S.; Wong, K.Y. Surface Modulated Fe Dopin g of β-Ni(OH)2 Nanosheets for Highly Promoted Oxygen Evolution Electrocatalysis. EcoMat 2022, 4, e12256. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, F.; Yuan, D.; Zhang, L.; Chen, Q.; Hong, M. Electric-Field Assisted in Situ Hydrolysis of Bulk Metal-Organic Frameworks (MOFs) into Ultrathin Metal Oxyhydroxide Nanosheets for Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2020, 59, 13101–13108. [Google Scholar] [CrossRef]
- Wu, X.; Song, X.; Tan, H.; Kang, Y.; Zhao, Z.; Jin, S.; Chang, X. Deciphering the Structure Evolution and Active Origin for Electrochemical Oxygen Evolution over Ni3S2. Mater. Today Energy 2022, 26, 101008. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, C.; Xu, L.; Lu, C.; Yang, J.; Pang, H.; Hou, W. Encapsulation of Janus-Structured Ni/Ni2P Nanoparticles within Hierarchical Wrinkled N-Doped Carbon Nanofibers: Interface Engineering Induces High-Efficiency Water Oxidation. Appl. Catal. B Environ. 2021, 298, 120578. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, Y.; Zhou, X.; Ye, Y.; Nie, K.; Wang, J.; Xie, M.; Zhang, Z.; Liu, Z.; Cheng, T.; et al. Promoting Nickel Oxidation State Transitions in Single-Layer NiFeB Hydroxide Nanosheets for Efficient Oxygen Evolution. Nat. Commun. 2022, 13, 6094. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Lee, S.; Kim, J.H.; Yoon, M.-H.; Lee, D.; Yoo, S.J. Unraveling Concentration-Dependent Solvation Structures and Molecular Interactions in Water-in-Salt Electrolytes for Enhanced Performance of Electric Double-Layer Capacitors. Energy Storage Mater. 2024, 65, 103137. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Ma, L.; Zhou, B.; Wu, J.; Zhu, D.; Li, Y.Y.; Fan, J.; Zhi, C. Aqueous Rechargeable Zinc Air Batteries Operated at −110 °C. Chem 2023, 9, 497–510. [Google Scholar] [CrossRef]
- Stevens, M.B.; Enman, L.J.; Batchellor, A.S.; Cosby, M.R.; Vise, A.E.; Trang, C.D.M.; Boettcher, S.W. Measurement Techniques for the Study of Thin Film Heterogeneous Water Oxidation Electrocatalysts. Chem. Mater. 2016, 29, 120–140. [Google Scholar] [CrossRef]
- Gorlin, M.; Chernev, P.; Ferreira de Araujo, J.; Reier, T.; Dresp, S.; Paul, B.; Krahnert, R.; Dau, H.; Strasser, P. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni-Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef]
- Costa, P.; Sandrin, D.; Scaiano, J.C. Real-Time Fluorescence Imaging of a Heterogeneously Catalysed Suzuki-Miyaura Reaction. Nat. Catal. 2020, 3, 427–437. [Google Scholar] [CrossRef]
- Do, V.-H.; Prabhu, P.; Li, Y.; Xie, W.; Kidkhunthod, P.; Wang, G.; Wang, X.; Lee, J.-M. Surface Activation of Atomically Thin Metal Nitride by Confined Nanoclusters to Trigger pH-Universal Hydrogen Evolution. Joule 2023, 7, 2118–2134. [Google Scholar] [CrossRef]
- Li, Y.; Bo, T.; Zuo, S.; Zhang, G.; Zhao, X.; Zhou, W.; Wu, X.; Zhao, G.; Huang, H.; Zheng, L.; et al. Reversely Trapping Isolated Atoms in High Oxidation State for Accelerating the Oxygen Evolution Reaction Kinetics. Angew. Chem. Int. Ed. 2023, 62, e202309341. [Google Scholar] [CrossRef]
- Tariq, I.; Asghar, M.A.; Ali, A.; Badshah, A.; Abbas, S.M.; Iqbal, W.; Zubair, M.; Haider, A.; Zaman, S. Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction. Catalysts 2022, 12, 1242. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Q.; Yu, J.; Zhang, X.; Wu, C.; Ma, Y.; An, H.; Wang, H.; Zhang, J.; Wang, X.; et al. Fundamental Understanding of Structural Reconstruction Behaviors in Oxygen Evolution Reaction Electrocatalysts. Adv. Energy Mater. 2023, 13, 2301391. [Google Scholar] [CrossRef]
- Cao, J.; Mou, T.; Mei, B.; Yao, P.; Han, C.; Gong, X.; Song, P.; Jiang, Z.; Frauenheim, T.; Xiao, J.; et al. Improved Electrocatalytic Activity and Stability by Single Iridium Atoms on Iron-Based Layered Double Hydroxides for Oxygen Evolution. Angew. Chem. Int. Ed. 2023, 62, e202310973. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bediako, D.K.; Hadt, R.G.; Hayes, D.; Kempa, T.J.; von Cube, F.; Bell, D.C.; Chen, L.X.; Nocera, D.G. Influence of Iron Doping on Tetravalent Nickel Content in Catalytic Oxygen Evolving Films. Proc. Natl. Acad. Sci. USA 2017, 114, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast Room-Temperature Synthesis of Porous S-Doped Ni/Fe (Oxy)Hydroxide Electrodes for Oxygen Evolution Catalysis in Seawater Splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M.; Lee, L.Y.S. Best Practices in Using Foam-Type Electrodes for Electrocatalytic Performance Benchmark. ACS Energy Lett. 2020, 5, 3260–3264. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Yue, L.; Dong, K.; Li, J.; Zhao, D.; Li, Z.; Sun, S.; Luo, Y.; Liu, Q.; et al. Benzoate Anions-Intercalated NiFe-layered Double Hydroxide Nanosheet Array with Enhanced Stability for Electrochemical Seawater Oxidation. Nano Res. Energy 2022, 1, e9120028. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhu, Q.; McElhenny, B.; Zhang, F.; Wu, C.; Xing, X.; Bao, J.; Chen, S.; Ren, Z. Boron-modified Cobalt Iron Layered Double Hydroxides for High Efficiency Seawater Oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational Design of Core-shell-structured CoP @FeOOH for Efficient Seawater Electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Qiu, J. Energy-Saving Hydrogen Production by Seawater Electrolysis Coupling Sulfion Degradation. Adv. Mater. 2022, 34, e2109321. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, Q.; Yu, L.; Duan, D.; Cao, T.; Qiu, S.; Wang, Z.; Guo, J.; Xie, Y.; Li, L.; et al. Functional Bimetal Co-Modification for Boosting Large-Current-Density Seawater Electrolysis by Inhibiting Adsorption of Chloride Ions. Adv. Energy Mater. 2023, 13, 2301475. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2020, 31, 2006484. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.; Xu, C.; Yin, Z. Heterogeneous Bimetallic Sulfides Based Seawater Electrolysis towards Stable Industrial-level Large Current Density. Appl. Catal. B Environ. 2021, 291, 120071. [Google Scholar] [CrossRef]
- Lan, C.; Xie, H.; Wu, Y.; Chen, B.; Liu, T. Nanoengineered, Mo-Doped, Ni3S2 Electrocatalyst with Increased Ni-S Coordination for Oxygen Evolution in Alkaline Seawater. Energy Fuels 2022, 36, 2910–2917. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble Metal-nitride Based Electrocatalysts for High-performance Alkaline Seawater Electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, Highly Sustained Splitting of Seawater into Hydrogen and Oxygen Fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Li, J.; Zhang, K.; Xia, Y.; Hui, B.; Yang, D. Wood Aerogel-derived Sandwich-like Layered Nanoelectrodes for Alkaline Overall Seawater Electrosplitting. Appl. Catal. B Environ. 2021, 293, 120215. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, S. A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting. Catalysts 2024, 14, 81. https://doi.org/10.3390/catal14010081
Wang H, Chen S. A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting. Catalysts. 2024; 14(1):81. https://doi.org/10.3390/catal14010081
Chicago/Turabian StyleWang, Haiming, and Sheng Chen. 2024. "A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting" Catalysts 14, no. 1: 81. https://doi.org/10.3390/catal14010081
APA StyleWang, H., & Chen, S. (2024). A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting. Catalysts, 14(1), 81. https://doi.org/10.3390/catal14010081