Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Analysis
2.2. Morphology
2.3. Chemical Compositions
2.4. Photocatalytic Performance
2.5. Charge Generation and Transfer
2.6. The Role of Bi in the Enhanced Production of Reactive Species
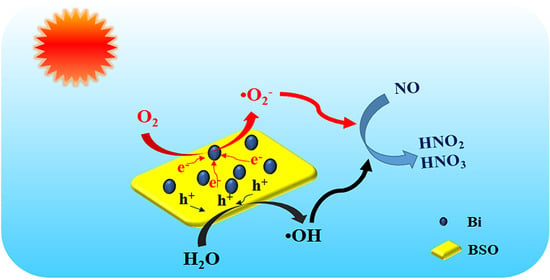
2.7. Analysis of the Photocatalytic Mechanism
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of Photocatalysts
3.3. Catalyst Characterization
3.4. Photocatalytic NO Removal Performance Test
3.5. Scavenging Experiments of Active Species
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chi, X.; Mandal, L.; Liu, C.; Fauzi, A.D.; Chaudhuri, A.; Whitcher, T.J.; Jani, H.K.; Chen, Z.; Xi, S.; Diao, C.; et al. Unravelling a new many-body large-hole polaron in a transition metal oxide that promotes high photocatalytic activity. NPG Asia Mater. 2022, 14, 61–67. [Google Scholar] [CrossRef]
- Li, F.; Liu, G.; Liu, F.; Yang, S. A review of self-cleaning photocatalytic surface: Effect of surface characteristics on photocatalytic activity for NO. Environ. Pollut. 2023, 327, 121580. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Olatunji, O.S. Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle-like SnO2 nanoparticles anchored on exfoliated g-C3N4. Environ. Sci. Eur. 2022, 34, 931–943. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. The monolithic transition metal oxide crossed nanosheets used for diesel soot combustion under gravitational contact mode. Appl. Surf. Sci. 2017, 406, 245–253. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Wang, H.; Cui, W.; Li, J.; Wang, J.; Yuan, C.; Zhuo, L.; Zhang, Y.; Dong, F. An atomic insight into BiOBr/La2Ti2O7 p-n heterojunctions: Interfacial charge transfer pathway and photocatalysis mechanism. Catal. Sci. Technol. 2020, 10, 826–834. [Google Scholar] [CrossRef]
- Zhu, P.; Yin, X.; Gao, X.; Dong, G.; Xu, J.; Wang, C. Enhanced photocatalytic NO removal and toxic NO2 production inhibition over ZIF-8-derived ZnO nanoparticles with controllable amount of oxygen vacancies. Chin. J. Catal. 2021, 42, 175–183. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Simultaneously efficient light absorption and charge transport of phosphate and oxygen-vacancy confined in bismuth tungstate atomic layers triggering robust solar CO2 reduction. Nano Energy 2017, 32, 359–366. [Google Scholar] [CrossRef]
- Zha, R.; Niu, Y.; Liu, C.; He, L.; Zhang, M. Oxygen vacancy configuration in confined BiVO4-Bi2S3 heterostructures promotes photocatalytic oxidation of NO. J. Environ. Chem. Eng. 2021, 9, 106586. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wan, Z.; Liu, Z.; Qian, J.; Deng, Y.; Zhang, S.; Zhong, Q. Efficient photothermal-assisted photocatalytic NO removal on molecular cobalt phthalocyanine/Bi2WO6 Z-scheme heterojunctions by promoting charge transfer and oxygen activation. Appl. Catal. B-Environ. 2022, 317, 121787. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Du, R.; Hu, X.; Wang, H.; Huang, J.; Xiang, Y.; Song, H.; Cai, Y.; Li, Z.; et al. Insights into the atomic structure of oxygen vacancy on Bi2MoO6/MXene heterojunction and its role for boosting photocatalytic NO oxidation. Appl. Surf. Sci. 2023, 638, 158104. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Ho, W.; Cao, J.; Shen, Z.; Lee, S.C. Fabrication of Bi2O2CO3/g-C3N4 heterojunctions for efficiently photocatalytic NO in air removal: In-situ self-sacrificial synthesis, characterizations and mechanistic study. Appl. Catal. B-Environ. 2016, 199, 123–133. [Google Scholar] [CrossRef]
- Liao, J.; Li, K.; Ma, H.; Dong, F.; Zeng, X.; Sun, Y. Oxygen vacancies on the BiOCl surface promoted photocatalytic complete NO oxidation via superoxide radicals. Chin. Chem. Lett. 2020, 31, 2737–2741. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Cui, H.; Wang, T. Preparation of direct Z-scheme Bi2Sn2O7/g-C3N4 composite with enhanced photocatalytic performance. J. Photochem. Photobiol. A-Chem. 2017, 335, 130–139. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Cui, X.; Lu, Y.; Yu, H.; Qin, W.; Huo, M. Visible-light activation of persulfate by a Z-scheme photocatalyst Fe-C3N4/Bi2Sn2O7 for tetracycline degradation. Appl. Catal. A-Gen. 2023, 666, 119422. [Google Scholar] [CrossRef]
- Fu, Q.; He, T.; Li, J.L.; Yang, G.W. Surface effect on electronic and optical properties of Bi2Ti2O7 nanowires for visible light photocatalysis. J. Appl. Phys. 2012, 111, 449–471. [Google Scholar] [CrossRef]
- Ren, J.; Liu, G.; Wang, Y.; Shi, Q. A novel method for the preparation of Bi2Ti2O7 pyrochlore. Mater. Lett. 2012, 76, 184–186. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Men, Q.; Ma, W.; Liu, Z.; Liu, Y.; Ma, C.; Huo, P.; Yan, Y. Photocatalytic removal using g-C3N4 quantum dots/Bi2Ti2O7 composites. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 213, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Cao, T.-P.; Zhao, Y.-H.; Sun, D.-W.; Wang, X. Preparation of Bi@Bi2Sn2O7/TiO2 Plasmonic Composite Fibers with Enhanced Photocatalytic Hydrogen Generation Activity. Chin. J. Inorg. Chem. 2019, 35, 1371–1378. [Google Scholar]
- Zhang, Y.; Di, J.; Qian, X.; Ji, M.; Tian, Z.; Ye, L.; Zhao, J.; Yin, S.; Li, H.; Xia, J. Oxygen vacancies in Bi2Sn2O7 quantum dots to trigger efficient photocatalytic nitrogen reduction. Appl. Catal. B-Environ. 2021, 299, 931–943. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Qin, Y.; Song, F.; Du, C.; Su, Y. Direct Z-scheme SnO2/Bi2Sn2O7 photocatalyst for antibiotics removal: Insight on the enhanced photocatalytic performance and promoted charge separation mechanism. J. Photochem. Photobiol. A-Chem. 2021, 404, 449–471. [Google Scholar] [CrossRef]
- Xu, W.; Fang, J.; Chen, Y.; Lu, S.; Zhou, G.; Zhu, X.; Fang, Z. Novel heterostructured Bi2S3/Bi2Sn2O7 with highly visible light photocatalytic activity for the removal of rhodamine B. Mater. Chem. Phys. 2015, 154, 30–37. [Google Scholar] [CrossRef]
- Dejpasand, M.T.; Saievar-Iranizad, E.; Bayat, A.; Ardekani, S.R. Surface plasmon-induced photodegradation of methylene blue with single layer graphene quantum dots/Au nanospheres under visible-light irradiation. J. Alloys Compd. 2021, 885, 931–943. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Nath, S.; Bapat, R.D.; Sharma, R.K.; Jagannath, B.; Dutta, B.; Hassan, P.A.; Tripathi, A.K. Superior Interfacial Contact Yields Efficient Electron Transfer Rate and Enhanced Solar Photocatalytic Hydrogen Generation in M/C3N4 Schottky Junctions. ACS Appl. Mater. Interfaces 2023, 15, 39926–39945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, B.; Pang, Y.; Sun, M.; Liu, S.; Fang, W.; Chen, L. Fabrication of 0D/1D Bi2MoO6/Bi/TiO2 heterojunction with effective interfaces for boosted visible-light photocatalytic degradation of tetracycline. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 638, 449–471. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Goharshadi, E.K. Decoration of graphene oxide as a cocatalyst on Bi doped g-C3N4 photoanode for efficient solar water splitting. J. Electroanal. Chem. 2022, 904, 13576–13582. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, B.; Li, Q.; Tian, C.; Ma, L.; Li, Z. The Application of Bi-Doped TiO2 for the Photocatalytic Oxidation of Formaldehyde. Cryst. Res. Technol. 2022, 57, 931–943. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Qiu, T.; Yang, L.; Han, Q.; Shen, Q.; Zhou, X.; Zhou, Y.; Zou, Z. Bismuth Vacancy-Induced Efficient CO2 Photoreduction in BiOCl Directly from Natural Air: A Progressive Step toward Photosynthesis in Nature. Nano Lett. 2021, 21, 10260–10266. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Lv, K.; Lu, Y.; Li, Q.; Wu, X.; Li, Y.; Li, X.; Fan, J.; Li, M. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Chin. J. Catal. 2019, 40, 755–764. [Google Scholar] [CrossRef]
- Xin, Y.; Zhu, Q.; Gao, T.; Li, X.; Zhang, W.; Wang, H.; Ji, D.; Huang, Y.; Padervand, M.; Yu, F.; et al. Photocatalytic NO removal over defective Bi/BiOBr nanoflowers: The inhibition of toxic NO2 intermediate via high humidity. Appl. Catal. B-Environ. 2023, 324, 122238. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Zhou, Z.; Tong, H.; Long, B.; Liu, W.; Li, W. Tailoring hydrophily and composition of BiOI for an ultrafast photodegradation of tetracycline hydrochloride. J. Environ. Chem. Eng. 2021, 9, 13576–13582. [Google Scholar] [CrossRef]
- Guo, S.; Di, J.; Chen, C.; Zhu, C.; Duan, M.; Lian, C.; Ji, M.; Zhou, W.; Xu, M.; Song, P.; et al. Oxygen vacancy mediated bismuth stannate ultra-small nanoparticle towards photocatalytic CO2-to-CO conversion. Appl. Catal. B-Environ. 2020, 276, 119156. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Z.; Shi, T.; Yang, S.; Zhang, Y. Atomically thin Bi/Bi4Ti3O12 heterojunction mediated increasing active photogenerated carriers for boosting photocatalytic activity. J. Colloid Interface Sci. 2022, 613, 625–635. [Google Scholar] [CrossRef]
- Puga, F.; Navio, J.A.; Cordoba, J.M.; Romero-Sarria, F.; Hidalgo, M.C. Insights into the structural and physicochemical properties of Zn-Bi-O composites for efficient photodegradation of caffeic acid, rhodamine B and methyl orange. Appl. Surf. Sci. 2022, 581, 931–943. [Google Scholar] [CrossRef]
- Yang, Y.; Bian, Z.; Zhang, L.; Wang, H. Bi@BiOx(OH)y modified oxidized g-C3N4 photocatalytic removal of tetracycline hydrochloride with highly effective oxygen activation. J. Hazard. Mater. 2022, 427, 931–943. [Google Scholar] [CrossRef]
- Fenelon, E.; You, S.-J.; Wang, Y.-F. Photodegradation of Nitrogen Oxide under Solar Light Using a Facile Synthesis Catalyst. Aerosol Air Qual. Res. 2021, 21, 931–943. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Zhao, Y.; Wu, J.; Li, X.; Qiao, Z.; He, P.; Qi, X.; Liu, Z.; Wei, G. Constructing 3D Bi/Bi4O5I2 microspheres with rich oxygen vacancies by one-pot solvothermal method for enhancing photocatalytic activity on mercury removal. Chem. Eng. J. 2021, 425, 449–471. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, H.; Yan, J.; Yan, L.; Li, K.; Guo, H.; Gong, W.; Lin, J. Highly efficient visible-light-driven photocatalytic hydrogen production on Cu7S4/Zn0.2Cd0.8S p-n binary heterojunctions. Int. J. Hydrogen Energy 2023, 48, 2171–2185. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Yang, F.; Liu, Y.; Chen, J.; Zhang, P.; Li, X.; Chen, X. Facile fabrication of TaON/Bi2MoO6 core-shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhang, W.; Gao, C.; Zhang, Y.; Dong, F. Plasmonic Bi metal as cocatalyst and photocatalyst: The case of Bi/(BiO)2CO3 and Bi particles. J. Colloid Interface Sci. 2017, 485, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Hao, J.; Zheng, S.; Yang, Y.; Yao, T.; Wang, Y. Construction of Z-scheme heterojunction by coupling Bi2Sn2O7 and BiOBr with abundant oxygen vacancies: Enhanced photodegradation performance and mechanism insight. J. Colloid Interface Sci. 2022, 612, 550–561. [Google Scholar] [CrossRef]
- Yin, N.; Chen, H.; Yuan, X.; Zhang, Y.; Zhang, M.; Guo, J.; Zhang, Y.; Qiao, L.; Liu, M.; Song, K. Highly efficient photocatalytic degradation of norfloxacin via Bi2Sn2O7/PDIH Z-scheme heterojunction: Influence and mechanism. J. Hazard. Mater. 2022, 436, 129317. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Chang, X.; Gong, J. Effective Charge Carrier Utilization in Photocatalytic Conversions. Acc. Chem. Res. 2016, 49, 911–921. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, C.; Zhong, J.; Li, J.; Huang, S. In-situ fabrication of Bi0/BiVO4 photocatalysts with boosted photocatalytic activity. Mater. Lett. 2022, 306, 130802. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, P.; Liu, M.; Xu, J.; Duan, M.; Luo, D. Synthesis and characterization of magnetic ZnFe2O4/Bi0-Bi2MoO6 with Z-scheme heterojunction for antibiotics degradation under visible light. Sep. Purif. Technol. 2021, 277, 119339. [Google Scholar] [CrossRef]
- Tan, S.T.; Lim, F.S.; Lee, W.J.; Lee, H.B.; Hong, K.J.; Oleiwi, H.F.; Chang, W.S.; Yap, C.C.; Jumali, M.H.H. Rational design of ordered Bi/ZnO nanorod arrays: Surface modification, optical energy band alteration and switchable wettability study. J. Mater. Res. Technol.-JMR&T 2021, 15, 5213–5220. [Google Scholar]
- Chen, Q.; Long, H.; Chen, M.; Rao, Y.; Li, X.; Huang, Y. In situ construction of biocompatible Z-scheme α-Bi2O3/CuBi2O4 heterojunction for NO removal under visible light. Appl. Catal. B-Environ. 2020, 272, 119008. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, Z.; Ke, J.; Xiao, H.; Hou, Y. In situ growing of Bi/Bi2O2CO3 on Bi2WO6 nanosheets for improved photocatalytic performance. Catal. Today 2018, 314, 2–9. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Li, J.; Jiang, G.; Zhou, Y.; Lee, S.; Dong, F. Transformation pathway and toxic intermediates inhibition of photocatalytic NO removal on designed Bi metal@defective Bi2O2SiO3. Appl. Catal. B-Environ. 2019, 241, 187–195. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, C.; Chen, Y.; Peng, Z.; Yang, J.; Zhang, Y. Simple Fabrication of Visible Light-Responsive Bi-BiOBr/BiPO4 Heterostructure with Enhanced Photocatalytic Activity. J. Nanomater. 2021, 2021, 931–943. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zhao, W.; Zhang, J.; Liu, X.; Gao, Y.; Ge, L. Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal. Catalysts 2024, 14, 275. https://doi.org/10.3390/catal14040275
Li N, Zhao W, Zhang J, Liu X, Gao Y, Ge L. Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal. Catalysts. 2024; 14(4):275. https://doi.org/10.3390/catal14040275
Chicago/Turabian StyleLi, Ning, Wenwen Zhao, Jiatong Zhang, Xuhui Liu, Yangqin Gao, and Lei Ge. 2024. "Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal" Catalysts 14, no. 4: 275. https://doi.org/10.3390/catal14040275
APA StyleLi, N., Zhao, W., Zhang, J., Liu, X., Gao, Y., & Ge, L. (2024). Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal. Catalysts, 14(4), 275. https://doi.org/10.3390/catal14040275