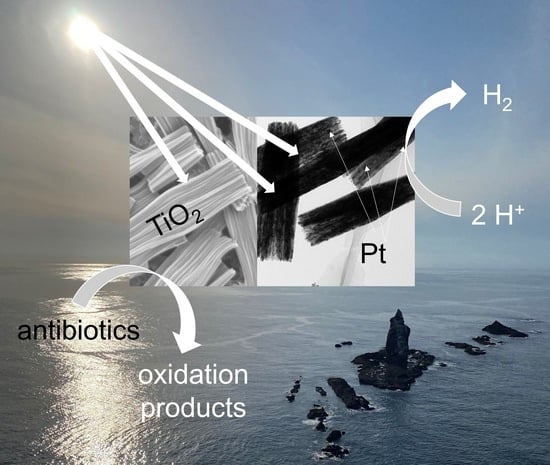
Platinum-Modified Rod-like Titania Mesocrystals with Enhanced Photocatalytic Activity
Abstract
:1. Introduction
- (i)
- Wide bandgap (ca. 3.20 eV), though profitable for good redox properties, also results in low light harvesting efficiency—only the UV range of the solar spectrum can be used;
- (ii)
- Recombination of charge carriers (typical for all semiconductors);
- (iii)
- Slow migration (transfer) of generated charge carriers (resulting from charge carriers’ recombination, permanent trapping in deep electron traps, amorphous phase content, irregular structure, etc.).
2. Results and Discussion
2.1. Structural Characterization of Unmodified Rod-like Titania Mesocrystals
2.2. Characterization of the Pt-Modified Rod-like Titania Mesocrystals
2.3. Photocatalytic Performance Evaluation
3. Experimental Section
3.1. Materials
3.2. Synthesis of Rod-like Titania Mesocrystals
3.3. Synthesis of Pt-Modified Rod-like Titania Mesocrystals
3.4. Physicochemical and Photocatalytic Characterization of Obtained Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chen, S.S.; Qi, Y.; Hisatomi, T.; Ding, Q.; Asai, T.; Li, Z.; Ma, S.S.K.; Zhang, F.X.; Domen, K.; Li, C. Efficient Visible-Light-Driven Z-Scheme Overall Water Splitting Using a MgTa2O6-xNy/TaON Heterostructure Photocatalyst for H-2 Evolution. Angew. Chem. Int. Edit 2015, 54, 8498–8501. [Google Scholar] [CrossRef]
- Ogawa, K.; Suzuki, H.; Zhong, C.; Sakamoto, R.; Tomita, O.; Saeki, A.; Kageyama, H.; Abe, R. Layered Perovskite Oxyiodide with Narrow Band Gap and Long Lifetime Carriers for Water Splitting Photocatalysis. J. Am. Chem. Soc. 2021, 143, 8446–8453. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Higashi, M.; Tomita, O.; Ishii, Y.; Yamamoto, T.; Kato, D.; Kotani, T.; Ozaki, D.; Nozawa, S.; Nakashima, K.; et al. PbBi3O4X3 (X = Cl, Br) with Single/Double Halogen Layers as a Photocatalyst for Visible-Light-Driven Water Splitting: Impact of a Halogen Layer on the Band Structure and Stability. Chem. Mat. 2021, 33, 9580–9587. [Google Scholar] [CrossRef]
- Kim, E.S.; Nishimura, N.; Magesh, G.; Kim, J.Y.; Jang, J.W.; Jun, H.; Kubota, J.; Domen, K.; Lee, J.S. Fabrication of CaFe2O4/TaON Heterojunction Photoanode for Photoelectrochemical Water Oxidation. J. Am. Chem. Soc. 2013, 135, 5375–5383. [Google Scholar] [CrossRef]
- Verma, P.; Yuan, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of plasmonic activity by Pt/Ag bimetallic nanocatalyst supported on mesoporous silica in the hydrogen production from hydrogen storage material. Appl. Catal. B-Environ. 2018, 223, 10–15. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B-Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are titania photocatalysts and titanium implants safe? Review on the toxicity of titanium compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. Fundamentals of TiO2 Photocatalysis. Consequences for Some Environmental Applications. In Heterogeneous Photocatalysis: From Fundamentals to Green Applications; Colmenares, J.C., Xu, Y.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 321–359. [Google Scholar]
- Gottuso, A.; De Pasquale, C.; Livraghi, S.; Palmisano, L.; Diré, S.; Ceccato, R.; Parrino, F. Nitrate radicals generated by TiO2 heterogeneous photocatalysis: Application to the cleavage of C=C double bond to carbonyl compounds. Mol. Catal. 2023, 550, 113607. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide photocatalysts active in visible light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef]
- Zabek, P.; Kisch, H. Polyol-derived carbon-modified titania for visible light photocatalysis. J. Coor. Chem. 2010, 63, 2715–2726. [Google Scholar] [CrossRef]
- Kisch, H.; Sakthivel, S.; Janczarek, M.; Mitoraj, D. A low-band gap, nitrogen-modified titania visible-light photocatalyst. J. Phys. Chem. C 2007, 111, 11445–11449. [Google Scholar] [CrossRef]
- Macyk, W.; Burgeth, G.; Kisch, H. Photoelectrochemical properties of platinum(IV) chloride surface modified TiO2. Photochem. Photobiol. Sci. 2003, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Trochowski, M.; Kobielusz, M.; Pucelik, B.; Dąbrowski, J.M.; Macyk, W. Dihydroxyanthraquinones as stable and cost-effective TiO2 photosensitizers for environmental and biomedical applications. J. Photochem. Photobiol. A Chem. 2023, 438, 114517. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T. Preparation of visible light active S-doped TiO2 photocatalysts and their photocatalytic activities. Wat. Sci. Technol. 2004, 49, 159–163. [Google Scholar] [CrossRef]
- Ohno, T.; Miyamoto, Z.; Nishijima, K.; Kanemitsu, H.; Xueyuan, F. Sensitization of photocatalytic activity of S- or N-doped TiO2 particles by adsorbing Fe3+ cations. Appl. Catal. A Gen. 2006, 302, 62–68. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Huang, J.; Jing, X.X.; Yang, D.Y.; Lin, H.; Wang, Z.G.; Li, P.; Chen, Y. Oxygen-deficient black titania for synergistic/enhanced sonodynamic and photoinduced cancer therapy at near iInfrared-II biowindow. Acs Nano 2018, 12, 4545–4555. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Akanda, M.M.A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Is Black Titania a Promising Photocatalyst? Catalysts 2022, 12, 1320. [Google Scholar] [CrossRef]
- Fu, Y.; Janczarek, M. Polyaniline–Titanium Dioxide Heterostructures as Efficient Photocatalysts: A Review. Crystals 2023, 13, 1637. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Dong, E.; Zhang, X.; Zhang, W.; Wang, Q.; Xu, S.; Li, H. Synthesis of CaIn2S4/TiO2 heterostructures for enhanced UV–visible light photocatalytic activity. J. Alloys Compd. 2021, 885, 161027. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Zebrowska, J.; Kobylanski, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core–shell photocatalysts. J. Mat. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B-Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.S.; Kowalska, E.; Ohtani, B. Enhanced Photocatalytic Activity by Particle Morphology: Preparation, Characterization, and Photocatalytic Activities of Octahedral Anatase Titania Particles. Chem. Lett. 2014, 43, 346–348. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Mahaney, O.O.P.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly Active Titania Photocatalyst Particles of Controlled Crystal Phase, Size, and Polyhedral Shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between photocatalytic activities and structural and physical properties of titanium(IV) oxide powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for Crystal-Face-Dependent TiO2 Photocatalysis from Single-Molecule Imaging and Kinetic Analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Mahaney, O.O.P.; Amano, F.; Murakami, N.; Abe, R. What Are Titania Photocatalysts?—An Exploratory Correlation of Photocatalytic Activity with Structural and Physical Properties. J. Adv. Oxid. Technol. 2010, 13, 247–261. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z-What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef]
- Mino, L.; Zecchina, A.; Martra, G.; Rossi, A.M.; Spoto, G. A surface science approach to TiO2 P25 photocatalysis: An in situ FTIR study of phenol photodegradation at controlled water coverages from sub-monolayer to multilayer. Appl. Catal. B Environmen. 2016, 196, 135–141. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, L.; Yue, X.; Mu, H.; Li, Z.; Chang, Y.; Janczarek, M.; Juodkazis, S.; Kowalska, E. Titania nanoengineering towards efficient plasmonic photocatalysis: Mono- and bi-metal-modified mesoporous microballs built of faceted anatase. Appl. Catal. B Environ. 2024, 345, 123654. [Google Scholar] [CrossRef]
- Kowalski, D.; Mallet, J.; Thomas, S.; Rysz, J.; Bercu, B.; Michel, J.; Molinari, M. Self-organization of TiO2 nanotubes in mono-, di- and tri-ethylene glycol electrolytes. Electrochim. Acta 2016, 204, 287–293. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Wang, L.; Mogan, T.R.; Wang, K.; Takashima, M.; Ohtani, B.; Kowalska, E. Fabrication and Characterization of Inverse-Opal Titania Films for Enhancement of Photocatalytic Activity. ChemEngineering 2022, 6, 33. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. Slow photon-induced enhancement of photocatalytic activity of gold nanoparticle-incorporated titania in-verse opal. Chem. Lett. 2021, 50, 711–713. [Google Scholar] [CrossRef]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. A nanocomposite superstructure of metal oxides with effective charge transfer interfaces. Nat. Commun. 2014, 5, 3038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, H.L.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.Y.; Liu, Y.; Elzatahry, A.; Che, R.C.; Li, W.; et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, S.; Du, M.; Ye, X.; Wang, Y.; Ye, J. Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysts 2019, 9, 91. [Google Scholar] [CrossRef]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fujitsuka, M.; Majima, T. Development of tailored TiO2 mesocrystals for solar driven photocatalysis. J. Energy Chem. 2016, 25, 917–926. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Disdier, J.; Pichat, P. Photoassisted platinum deposition on TiO2 powder using various platinum complexes. J. Phys. Chem. 1986, 90, 6028–6034. [Google Scholar] [CrossRef]
- Ohtani, B.; Okugawa, Y.; Nishimoto, S.; Kagiya, T. Photocatalytic activity of titania powders suspended in aqueous silver nitrate solution: Correlation with pH-dependent surface structures. J. Phys. Chem. 1987, 91, 3550–3555. [Google Scholar] [CrossRef]
- Nishimoto, S.I.; Ohtani, B.; Kagiya, T. Photocatalytic Dehydrogenation of Aliphatic-Alcohols by Aqueous Suspensions of Platinized Titanium-Dioxide. J. Chem. Soc. Farad. Trans. 1 Phys. Chem. Condens. Phases 1985, 81, 2467–2474. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Takahashi, Y.; Tatsuma, T. Plasmon-resonance-based generation of cathodic photocurrent at electrodeposited gold nanoparticles coated with TiO2 films. Chem. Phys. Chem. 2009, 10, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Bielan, Z.; Kowalska, E.; Dudziak, S.; Wang, K.; Ohtani, B.; Zielinska-Jurek, A. Mono- and bimetallic (Pt/Cu) titanium(IV) oxide core–shell photocatalysts with UV/Vis light activity and magnetic separability. Catal. Today 2021, 361, 198–209. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Li, G.; An, T.; Yamashita, H. Fabrication of Au/TiO2 nanowires@carbon fiber paper ternary composite for visible-light photocatalytic degradation of gaseous styrene. Catal. Today 2017, 281, 621–629. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Kuppusami, P.; Parvathavarthini, N.; Mohandas, E. Pulsed laser deposition of anatase and rutile TiO2 thin films. Surf. Coat. Tech. 2007, 201, 7713–7719. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous Anatase TiO2 Mesocrystals: Additive-Free Synthesis, Remarkable Crystalline-Phase Stability, and Improved Lithium Insertion Behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef]
- Kelly, B.G.; Loether, A.B.; DiChiara, A.D.; Henning, R.W.; DeCamp, M.F.; Unruh, K.M. Lattice parameter evolution in Pt nanoparticles during photo-thermally induced sintering and grain growth. J. Phys. Chem. Solids 2017, 108, 104–108. [Google Scholar] [CrossRef]
- Wei, Z.S.; Endo-Kimura, M.; Wang, K.L.; Colbeau-Justin, C.; Kowalska, E. Influence of semiconductor morphology on photocatalytic activity of plasmonic photocatalysts: Titanate nanowires and octahedral anatase nanoparticles. Nanomaterials 2019, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Zhao, X.J.; Zhao, Q.N. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid. Films 2000, 379, 7–14. [Google Scholar] [CrossRef]
- Reszczynska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B-Environ. 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Reszczynska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Jensen, H.; Soloviev, A.; Li, Z.S.; Sogaard, E.G. XPS and FTIR investigation of the surface properties of different prepared titania nano-powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar] [CrossRef]
- Ono, L.K.; Yuan, B.; Heinrich, H.; Cuenya, B.R. Formation and Thermal Stability of Platinum Oxides on Size-Selected Platinum Nanoparticles: Support Effects. J. Phys. Chem. C 2010, 114, 22119–22133. [Google Scholar] [CrossRef]
- Zheng, X.; Chan, M.H.-Y.; Chan, A.K.-W.; Cao, S.; Ng, M.; Sheong, F.K.; Li, C.; Goonetilleke, E.C.; Lam, W.W.Y.; Lau, T.-C.; et al. Elucidation of the key role of Pt···Pt interactions in the directional self-assembly of platinum(II) complexes. Proc. Natl. Acad. Sci. USA 2022, 119, e2116543119. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Luo, E.; Yang, L.; Chu, Y.; Gao, L.; Jin, Z.; Liu, C.; Ge, J.; Xing, W. Stabilized Pt Cluster-Based Catalysts Used as Low-Loading Cathode in Proton-Exchange Membrane Fuel Cells. ACS Energy Lett. 2020, 5, 3021–3028. [Google Scholar] [CrossRef]
- Dvořák, F.; Farnesi Camellone, M.; Tovt, A.; Tran, N.-D.; Negreiros, F.R.; Vorokhta, M.; Skála, T.; Matolínová, I.; Mysliveček, J.; Matolín, V.; et al. Creating single-atom Pt-ceria catalysts by surface step decoration. Nature Commun. 2016, 7, 10801. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Marić, I.; Dražić, G.; Ivanda, M.; Jurkin, T.; Štefanić, G.; Gotić, M. Impact of Fe(III) ions on the structural and optical properties of anatase-type solid solutions. J. Mol. Struct. 2019, 1179, 354–365. [Google Scholar] [CrossRef]
- Nemashkalo, A.B.; Busko, T.O.; Peters, R.M.; Dmytrenko, O.P.; Kulish, M.P.; Vityuk, N.V.; Tkach, V.M.; Strzhemechny, Y.M. Electronic band structure studies of anatase TiO2 thin films modified with Ag, Au, or ZrO2 nanophases. Phys. Status Solidi B Basic. Res. 2016, 253, 1754–1764. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.H.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Stietz, F.; Trager, F. Surface plasmons in nanoclusters: Elementary electronic excitations and their applications. Philos. Mag. 1999, 79, 1281–1298. [Google Scholar] [CrossRef]
- Douketis, C.; Shalaev, V.M.; Haslett, T.L.; Wang, Z.; Moskovits, M. The role of localized surface plasmons in photoemission from silver films: Direct and indirect transition channels. J. Electron. Spectrosc. 1993, 64/65, 167–175. [Google Scholar] [CrossRef]
- Xia, Y.N.; Xiong, Y.J.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Edit. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Xia, Y.N.; Halas, N.J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005, 30, 338–344. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Kunwar, S.; Sui, M.; Pandey, P.; Gu, Z.; Pandit, S.; Lee, J. Improved Configuration and LSPR Response of Platinum Nanoparticles via Enhanced Solid State Dewetting of In-Pt Bilayers. Sci. Rep. 2019, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Tsounis, C.; Scott, J.; Low, G.K.-C.; Amal, R. Promoting Catalytic Oxygen Activation by Localized Surface Plasmon Resonance: Effect of Visible Light Pre-treatment and Bimetallic Interactions. Chemcatchem 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Tian, Z.; Wu, Y.; Cui, Y. Efficiently Visible-Light Driven Photoelectrocatalytic Oxidation of As(III) at Low Positive Biasing Using Pt/TiO2 Nanotube Electrode. Nanoscale Res. Lett. 2016, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Chelvayohan, M.; Mee, C.H.B. Work Function Measurements on (110), (100) and (111) Surfaces of Silver. J. Phys. C Solid. State 1982, 15, 2305–2312. [Google Scholar] [CrossRef]
- Sachtler, W.M.H.; Dorgelo, G.J.H.; Holscher, A.A. The work function of gold. Surf. Sci. 1966, 5, 221–229. [Google Scholar] [CrossRef]
- Sachtler, W.M.; Dorgelo, G.J.H. Surface of Copper-Nickel Alloy Films: I. Work Function and Phase Composition. J. Catal. 1965, 4, 654–664. [Google Scholar] [CrossRef]
- Chen, Y.; Soler, L.; Cazorla, C.; Oliveras, J.; Bastús, N.G.; Puntes, V.F.; Llorca, J. Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution. Nat. Commun. 2023, 14, 6165. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Ohtani, B.; Iwai, K.; Nishimoto, S.-i.; Sato, S. Role of Platinum Deposits on Titanium(IV) Oxide Particles: Structural and Kinetic Analyses of Photocatalytic Reaction in Aqueous Alcohol and Amino Acid Solutions. J. Phys. Chem. B 1997, 101, 3349–3359. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor-Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Kisch, H. On the Problem of Comparing Rates or Apparent Quantum Yields in Heterogeneous Photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Pellizzari, P.; Maurino, V.; Pelizzetti, E.; Vione, D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Appl. Catal. B-Environ. 2008, 77, 308–316. [Google Scholar] [CrossRef]
- Minero, C.; Maurino, V.; Pelizzetti, E.; Vione, D. An empirical, quantitative approach to predict the reactivity of some substituted aromatic compounds towards reactive radical species (Cl−2•, Br−2•, •NO2, SO3−•, SO4−•) in aqueous solution. Environ. Sci. Pollut. R. 2006, 13, 212–214. [Google Scholar]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, A.; Wojtyla, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, H.; Qi, J.; Huang, T.; Wang, C.-C.; Liu, W. Degradation of acetaminophen by activated peroxymonosulfate using Co(OH)2 hollow microsphere supported titanate nanotubes: Insights into sulfate radical production pathway through CoOH+ activation. Chem. Eng. J. 2021, 406, 126877. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic Titania Photocatalysts Active under UV and Visible-Light Irradiation: Influence of Gold Amount, Size, and Shape. J. Nanotechnol. 2012, 2012, 361853. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.; Pichat, P.; Fernandez, A.; Gonzalez-Elipe, A.; Munuera, G.; Leclercq, C. Titania-supported bimetallic catalyst synthesis by photocatalytic codeposition at ambient temperature: Preparation and characterization of platinum-rhodium, silver-rhodium, and platinum-palladium couples. J. Catal. 1991, 132, 490–497. [Google Scholar] [CrossRef]
- Hussein, F.H.; Rudham, R. Photocatalytic dehydrogenation of liquid alcohols by platinized anatase. J. Chem. Soc. Faraday Trans. 1 1987, 83, 1631–1639. [Google Scholar] [CrossRef]
- Tanaka, K.; Harada, K.; Murata, S. Photocatalytic deposition of metal ions onto titanium dioxide powder. Sol. Energy 1986, 36, 159–161. [Google Scholar] [CrossRef]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazi, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Ji, Y.; Bielan, Z.; Yue, X.; Xu, Y.; Sun, J.; Chen, S.; Yi, G.; Chang, Y.; Kowalska, E. Platinum-Modified Rod-like Titania Mesocrystals with Enhanced Photocatalytic Activity. Catalysts 2024, 14, 283. https://doi.org/10.3390/catal14040283
Wei Z, Ji Y, Bielan Z, Yue X, Xu Y, Sun J, Chen S, Yi G, Chang Y, Kowalska E. Platinum-Modified Rod-like Titania Mesocrystals with Enhanced Photocatalytic Activity. Catalysts. 2024; 14(4):283. https://doi.org/10.3390/catal14040283
Chicago/Turabian StyleWei, Zhishun, Yuanyuan Ji, Zuzanna Bielan, Xin Yue, Yuqi Xu, Jiajie Sun, Sha Chen, Guoqiang Yi, Ying Chang, and Ewa Kowalska. 2024. "Platinum-Modified Rod-like Titania Mesocrystals with Enhanced Photocatalytic Activity" Catalysts 14, no. 4: 283. https://doi.org/10.3390/catal14040283
APA StyleWei, Z., Ji, Y., Bielan, Z., Yue, X., Xu, Y., Sun, J., Chen, S., Yi, G., Chang, Y., & Kowalska, E. (2024). Platinum-Modified Rod-like Titania Mesocrystals with Enhanced Photocatalytic Activity. Catalysts, 14(4), 283. https://doi.org/10.3390/catal14040283